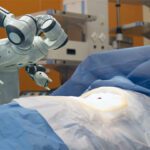
How to Choose a Medical Connector
Connectivity solutions for medical equipment must be safe, dependable, durable, easy to operate, and meet critical regulatory requirements.

Medical connectors are used to transmit data, power, and control signals to a system or piece of equipment that provides patient care in a hospital, clinic, or home health setting. There is more to specifying and choosing a medical connector than meeting technical requirements. The right connector can enhance equipment durability, ease-of-use, safety, and efficiency. High-quality interconnect components can also play a role in improving the effectiveness and end-user value of the associated medical equipment or systems. However, increased pressure from rising costs, complex regulatory requirements, and supply chain challenges continue to affect the medical connector industry.
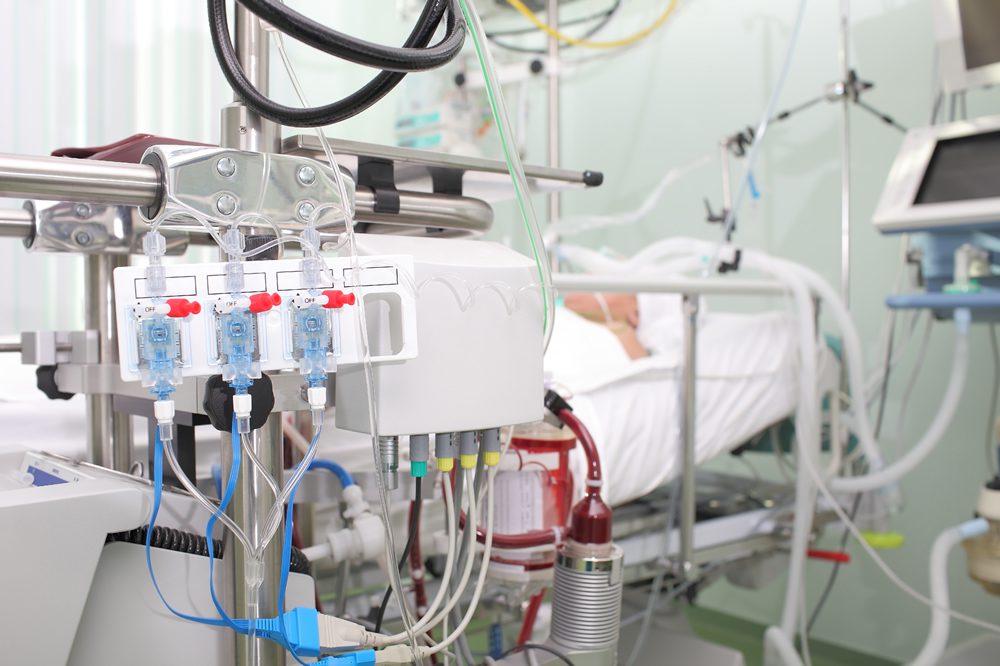
Specifying the right medical connector can enhance equipment durability, ease-of-use, safety, and efficiency.

The Viking Thorkom series, one of the original autoclavable interconnects, features high-performance materials that serve test instruments and industrial applications as well as thousands of medical devices. Its durability and ease of use make it a continued favorite for new medical designs.
Medical connector attributes
The convergence of technologies from other applications and industry trends creates a significant advantage for healthcare interconnectivity. Many proven designs and materials have crossed over from military, industrial, and aerospace applications. Well-planned designs assure interoperability between devices, clinicians, and delivery systems throughout the patient treatment experience.
Connector interfaces are the format of the connector contact surfaces (plug or receptacle) and their patterns. Minimizing footprint size is paramount in today’s applications, with the aim of having high power density in a small area. The most common interfaces for equipment interconnectivity are circular M8, M12, and M23 connectors, standard and micro D-sub rectangular connectors, Ethernet, and micro card-edge formats. While copper-wire type connectors (metal contacts terminated from copper-core wiring) continue to be a mainstay in medical technologies, fiber optic interconnects are also vital in many diagnostic and therapeutic applications.
Electrical operating signal type and capacity are vital attributes when reviewing interconnect requirements. While most equipment manufacturers will have predetermined signal type and minimal strength needed, optimizing interconnectivity may still need to be addressed. Connectors that are configurable for mixed signals or hybrid formats could be considered instead of a single-purpose interconnect to shrink the overall footprint. Low signal levels such as those common in cardiac monitoring equipment may also mandate EMC protections such as 360° EMC shielding to optimize the signal. And high-voltage interconnects rated for 1KV up to 100KV could be specified for transformers, diagnostic equipment, and laser therapy.

Mounting and mating methods must meet a myriad of technical and practical requirements, with color-coding identification as a popular method to ensure connector plugs are mated to the proper receptacles.
Mounting and mating methods must meet a myriad of technical and practical requirements. Robust connector-to-connector options include push-pull, quick disconnect, breakaway, bayonet, and screw mating; however, all require an ergonomic design and light weight. Blind mating brings benefits when connectivity lacks a clear line of sight. This connection method is ideal for use with gloved hands and equipment operators of all ability levels. Color-coding identification is also popular to ensure connector plugs are mated to the proper receptacles.
Connectors and handsets are often the most handled parts of medical equipment, requiring a unique and much higher level of durability than interconnects utilized in other industries or applications. Medical connectors require a high mating cycle rating of up to 10,000 mating cycles, especially in diagnostic and imaging equipment. Overmolding the connector to the cable, where applicable, provides significantly enhanced durability for high mating cycle interconnects.

Shell materials need to be medical-specific.
While interface and signal materials are frequently shared with non-healthcare applications, shell materials need to be medical-specific. The primary requirement for shell materials for medical connectors is the capability to be sterilized before reuse. The most common sterilization methods the shell needs to withstand are autoclaving, Ethylene Oxide (EtO), gamma, and chemical washdown. PPSU, PSU, and PEI are three popular thermoplastic high-performance plastics that combine highly favorable thermal, electrical, and mechanical/dimensional stability properties. Limiting the DEHP content of the connector shell and structural components is a safety requirement. To maintain durability, components must also resist energy-rich radiation-like X-rays.

A primary requirement for medical connectors is the capability to be sterilized before reuse, with the most common sterilization methods being autoclaving, Ethylene Oxide (EtO), gamma and chemical washdown.
Protecting the equipment from water and solids when the connectors will be utilized in a clinical setting, including dental facilities, requires ingress protection (IP) ratings of IP50, IP67, and IP68 — the most prevalent specification for medical connectors. However, an IP68 sealing rate is preferable, as it indicates that connectors are protected in both mated and unmated states. Surface mount seal ingress protection ratings are also vital to protect the equipment itself.
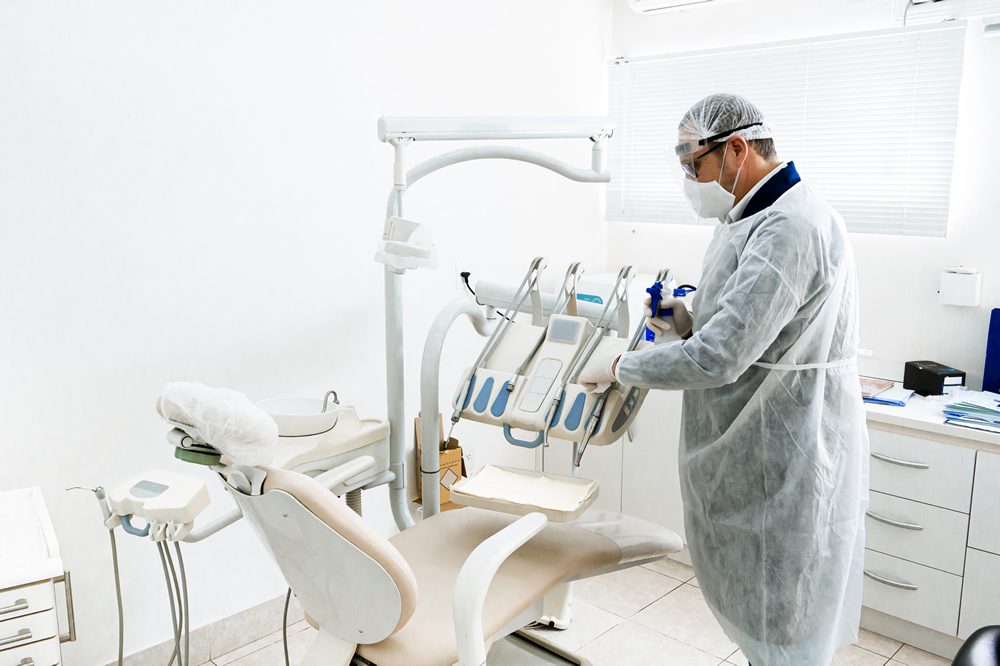
Water/solids ingress protection ratings are vital when the connectors will be utilized in a clinical setting, including dental facilities, with an IP68 sealing rate being preferable as connectors are protected in both mated and unmated states.
Overmolding options are highly preferable for cable-mounted interconnects, as this feature eliminates unnecessary grooves or cavities in transitional areas between the connector and cable. Smooth and highly stable overmolded transitions are highly preferable to compression-fit transitions; even if they are rated to resist splash and dust, overmolding provides an important layer of protection.
Medical connectors include interconnecting patient-facing devices and internal interconnects within the equipment itself. Optimum quality power card-edge connectors, power headers, input/output connectors, and RF interconnects are used in virtually all diagnostic, surgical, and patient monitoring equipment, as well as internal cable assemblies.
Medical connector standards and regulations
Most countries have highly specific sets of standards that need to be satisfied for medical connectors to be used in clinical settings. Standards such as US, CSA, and others guide the selection of components that will be able to endure the harsh environment conditions of medical environments and ensure critical patient and provider safety. Some quality protocols that may need to be satisfied include:
- UL or CSA approvals
- ISO 13485 certification
- NEMA approval
- FDA registration as a medical device manufacturer (assures FDA approval and oversights and shifts the burden of supplier regulatory compliance to the manufacturer)
- Japanese Ministry of Health accreditation
- CE marking under Medical Device Directive
- Color coding (mating surface) for similar connectors with different functions
Typical applications of medical connectors
Medical connectors support patient care, diagnoses, and monitoring in applications including portable devices, surgical robotics, diagnostics equipment, patient monitoring devices, dental surgery equipment, and plasma medicine. The monitor-end connectors used in these devices may feature unique keying and color-coding to facilitate connection and reduce errors. Robotic-assisted devices require high-speed, durability, and reliability in interconnects for both copper and fiber optic final cable assemblies.
Ambulatory monitoring requires smaller, lighter, and integrated interconnect solutions, especially for telehealth applications. Remote/mobile and field testing and remote diagnostic applications require a unique combination of ruggedization and precision with the highest water and particulate IP ratings.
Why many distributors don’t focus on single-use connectors
Single-use connectors don’t need to meet the rigorous sterilization requirements that shape many connectors used in the medical industry. However, this market segment is highly cost-dependent, the interconnects tend to have limited capabilities, and many are application-specific. Single-use connectors typically support general purpose wearables, continuous glucose monitoring systems, and other biopharma applications.
The future of medical connectors
New interconnect development must keep up with the pace of innovation in the medical industry. The continuous development of applications requires new products and materials that will be faster and more efficient to produce. Combined interconnect power and signal capabilities in increasingly compact footprints are a significant area of recent development.

Medical connectors should not just be classified exclusively as interconnecting patient-facing devices but also as vital internal interconnects within the equipment.
Another macro-trend within the MedTech industry is electrophysiology, which combines diagnostics and treatment in a single procedure. As electrophysiology becomes a mainstream cardiac treatment, more complex interconnects will be needed to expand this type of treatment.
Versatility is another trend impacting the development of medical connectors. Products that merge attributes of single-use and reusable components have great potential for designers. While the strength of traditional single-use interconnects is minimizing the potential for cross contamination, those designs can be costly and have more limited signal and current capabilities. Materials science will be the key to cost containment and durability for these connectors.
Partnering with authorized manufacturers is a win/win
Distributors that partner with trusted medical connector manufacturers can leverage cross-industry expertise derived from other interconnect applications, minimizing time-to-market for new requirements. This enables end users to choose from the newest technologies and materials, and access components that meet the most recent industry-specific certifications.
Connectors and cables are becoming more advanced in enabling electronic equipment to operate safely and reliably. If one connector fails, it could render the whole device inoperable, including the entire electronic ecosystem that was integrated with the device. This makes it vital for connectivity solutions to be dependable in signal transmission, durable, robust in functionality, and meet critical regulatory requirements.
By CDM Electronics
Explore more solutions for medical device design at CDM Electronics.
Subscribe to our weekly e-newsletters, follow us on LinkedIn, Twitter, and Facebook, and check out our eBook archives for more applicable, expert-informed connectivity content.