Antimicrobial Interconnects Resist Germs, Infection
Whether implanted in the body or used against skin, patients depend on electronic devices to resist bacteria. A new generation of biocompatible materials makes high-tech medicine safer than ever.

Cardiac pacemakers, stents, knee and hip implants, insulin pumps, cochlear implants, and other electronic medical devices have increased the quality of life for millions of people. But introducing a new object or material into the human body also presents potential risks such as infection, allergic reaction, or irritation and rejection. It is critical that every aspect of a device, from package design to material composition, be as biocompatible as possible and resist the growth of microorganisms that could cause infection. In complex implantable or wearable electronic systems, antimicrobial interconnects contribute to the safety and functionality of devices.
Antimicrobial interconnects are not limited to the medical market. Food manufacturing and packaging equipment must also resist the growth of microbes and viruses to protect the safety of our food supply. Research facilities that require absolute sterility utilize antimicrobial interconnects. Space environments, such as the International Space System, must remain as sterile as possible. Microorganisms or microbes, including viruses, bacteria, algae, and fungi, can populate surfaces and materials. If these microbes enter difficult to access areas such as tubing or closed devices, they can populate and create dangerous conditions for the human body, research activities, or the safe production of food and medicines. Disposable interconnects are one solution used to reduce risk, but in implantable electronics, closed environments such as space, or remote medical clinics where equipment cannot easily be replaced, a reusable solution is required.
Antimicrobial materials integrate substances that resist or kill microbes. Copper, silver, titanium, stainless steel, and other metals display antibacterial, antiviral, and anti-fungal properties and are widely used in electronic devices, including shells, pins, and other metal components used in interconnects. In recent years, materials innovations have led to the development of antimicrobial polymers and plastic additives used in cables, overmolding, and connector shells. Connector and cable suppliers utilize these materials and also design interconnects with a form factor that resists microbe growth, such as eliminating cavities.
Materials with antimicrobial properties must also comply with safety and biocompatibility standards for electronic components to ensure that the substances don’t affect the human body. For example, polymers must not leach or impart endocrine disrupting chemicals. Regulations vary by region, market, and specific product use. Duration of contact, type of contact (blood, mucosal membranes), and category of contact (implant or surface) are among the factors that must be considered. UL, CSA, and CE requirements must also be met. Food grade and medical grade products are covered by ISO 10993-12 and 10993-18, and in Europe, the ROHS and REACH directives. In many components, biocompatibility and antimicrobial properties go hand in hand. Materials that have a chemical resistance to microbe growth must also be safe for human contact.
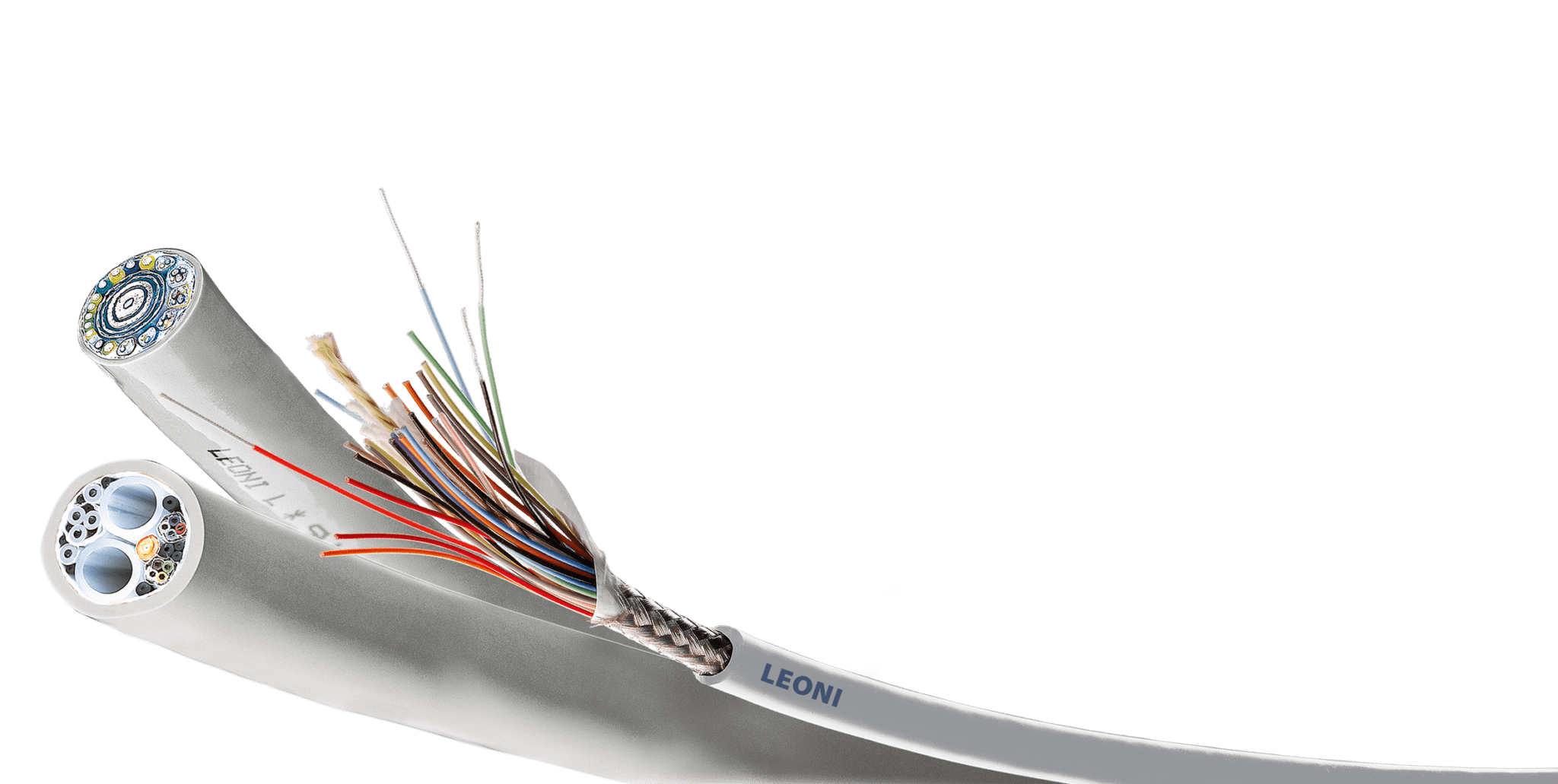
Antimicrobial endoscopy cables from LEONI, a BizLink company.
LEONI, a BizLink company, achieves biocompatibility for its cord and cable products, including its endoscopy cable system, through an acid-based technology that gives plastic surfaces a germ-killing effect. These antimicrobial cables have special properties that are similar to the principle of the human acid protective layer. “The method that LEONI applies follows the Lewis acid-base theory: it involves acid ions being released on the surface of the cables that lower the pH value on the outer surface of the jacket. This restricts the cell functions of the germs and their separation, so that they ultimately die. This is made possible by permanently incorporating a special metal oxide in variable doses in the plastic matrix of the cable jacket. Even in low concentration, a significant germ-killing effect is already evident. The mechanical properties of the cable itself and its handling remain unchanged,” said Holger Lücking, director of marketing, BizLink.

Medical cables and cable assemblies offered by Axon’ Cable are designed to be flexible and withstand handling from the medical staff and patients in applications including endoscopy, ambulatory equipment, patient monitoring, and diagnostics.
Axon’ Cable offers cables and cable assemblies which meet sterilization, biocompatibility, flexibility, and miniaturization requirements. For protection against contamination, Axon’ Cable has incorporated the technology of silver ion micro-flux into its NOSO-FREE antibacterial cable assemblies. This technology allows for inhibiting the growth of bacteria, virus, fungi, and other microbes. “E-coli bacteria grows at a rate of three generations per hour at 37 °C. In one hour, a population of 100 cells is multiplied by 8. In less than five hours, it will exceed one million. However, with NOSO-FREE materials, the number of bacteria, microbes, and viruses on the surface of equipment continually decreases from the first hour until their total elimination after 24 hours.

SCHURTER’s antimicrobial switches disinfect themselves through coatings that resist bacteria growth. These are suitable for use in medical and food processing equipment.
In addition to interconnects, other elements in a connected system can be contaminated through human touch or exposure to contaminated fluids. SCHURTER has developed antimicrobial switches and input systems (including touchscreens) that meet hygienic requirements for medical applications. Antibacterial glass, flexible, silver-coated polyester films, as well as surface-treated housings help ensure that devices resist bacteria growth.

L-com offers a range of antibacterial/antimicrobial Ethernet cables, available from Newark, that can be used in telecommunications and networking in medical, food, or other applications where harmful bacteria and other pathogens may contaminate cables and the surrounding environments with common bacteria such as Staphylococcus Aureus and E. coli.
However, all antimicrobial surfaces achieve only limited effectiveness. Package design elements that ensure that components can be disinfected as easily as possible also help reduce risks. A safe, effective, and compliant device requires a careful evaluation of every element, from materials to components to product housing. New materials and products are continually becoming available to make this process easier.
To learn more about the companies mentioned in this article, visit the Preferred Supplier pages for Axon’ Cable, BizLink, Newark, an Avnet Company, and SCHURTER.
Like this article? Check out our other articles on cable, cable assemblies, our Medical Market Page, and our 2022 Article Archive.
Subscribe to our weekly e-newsletters, follow us on LinkedIn, Twitter, and Facebook, and check out our eBook archives for more applicable, expert-informed connectivity content.
- Meet the Connector: DIN Standard Connectors - April 16, 2024
- Software-Driven Radio Reinvigorates Old Technology - April 9, 2024
- What is a Busbar? - April 2, 2024