Silver: A Superior Finish for High-Current Applications
In the next article in the “Connector Basics” series, Ed Bock of APEX Electrical Interconnection Consultants looks at the attributes of silver and explains why it’s a superior finish for high-current applications.
 Connector designers have a wide selection of finishes to consider for high-current applications, both noble as well as non-noble metals. It is important to evaluate performance as well as cost aspects. Failures of high-current contacts are somewhat distinct from those of signal contacts, which in that case are primarily associated with loss of information. In contrast, the failure of a high-current contact could result in something as catastrophic as a fire.
Connector designers have a wide selection of finishes to consider for high-current applications, both noble as well as non-noble metals. It is important to evaluate performance as well as cost aspects. Failures of high-current contacts are somewhat distinct from those of signal contacts, which in that case are primarily associated with loss of information. In contrast, the failure of a high-current contact could result in something as catastrophic as a fire.
Silver has the highest value of electrical conductivity of any material; clearly, this is a significant factor when considering the ability of a contact to carry current. Silver is “semi-noble,” in that although oxide formation is mitigated, it is susceptible to the formation of tarnish films. As with noble metals, silver will not degrade as a consequence of fretting corrosion. For electrical contacts, by utilizing the recommended normal forces and plating thicknesses, successful performance (in spite of tarnish formation) can be expected.
The contact interface of a high-current contact should remain relatively cool, certainly less than the softening/melting temperature of the contact metal such as silver. Silver softens at about 180°C and melts at about 960°C. An important parameter, I2/F, relates the required normal force to the current such that neither the softening nor the melting temperature at the interface will be reached. In essence, the value of I2/F becomes a measure of the ability of the contact finish to withstand heating, where I is current in amperes and F is force in newtons. The larger this value, the better the contact finish is at handling current. The following table lists information for a number of contact finish materials. Clearly, silver is by far the best choice.
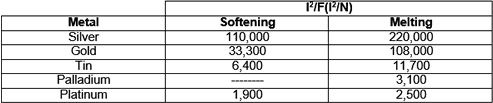
Softening and melting parameters for some contact finish materials.
As mentioned earlier, tarnish films will likely be the prime degradation mechanism for a silver-finished contact. With signal contacts and their generally lower values of normal force, as compared to high-current contacts, successful performance could be somewhat of a concern.
Tarnish films on silver are readily disrupted with moderate levels of contact normal force. These tarnish films are somewhat soft and penetrated/displaced with reasonable values of normal force. Contact studies performed by the Bell Telephone Laboratories reached the following conclusion: A force of 1 to 2N would ensure low and stable contact resistance in spite of sulfiding. We would therefore recommend that normal forces for high-current silver-plated contacts be on the order of several hundred grams.
An additional attribute associated with silver is its ability to self-heal; this means that silver has the ability to modify the contact interface and improve performance as degradation occurs. As a contact degrades, its contact resistance increases; with current, the contact interface will increase in temperature. At some point the interface temperature will reach the softening point of silver; with recommended normal forces, asperities at the contact interface will collapse on themselves thereby increasing the size of the contact interface. That will result in a reduction of the contact resistance. This same mechanism with a non-noble contact finish, such as tin, could lead to additional degradation and the possibility of thermal runaway.
The recommended plating thickness for silver is generally 125 microinches or more. This assures sufficient thickness of the silver to allow self-healing and addresses the concern that the durability properties of silver are somewhat limited. In order for the self-healing process to be effective, it requires that the tarnish not consume the total thickness of the plate. The use of a lubricant that would provide the benefits of increased durability and tarnish inhibition could be considered.
Generally, silver would be compatible with most contact finishes and intermateability should not be an issue – certainly the well-known warning and problems associated with mating gold to tin would not apply.
Among materials selected for contact finishes, silver is considered somewhat susceptible to electromigration. Under certain conditions, the outcome could be the formation of a metallic bridge between adjacent contacts, which would result in excessive leakage resistance and/or a potential short. Operation in a high humidity/moisture-condensing environment would be a concern. Conditions that support such a failure include closely spaced contacts (a few tens of millimeters or less) with a material path between them (such as a PCB), a DC potential difference between the contacts of about 1V, and the necessary high-humidity conditions. Consideration of the design and application parameters should indicate whether electromigration is a possible failure mechanism – if deemed warranted, a properly designed verification test should be conducted.
The advantages of using silver as a contact finish for high-current applications include:
- Highest conductivity, very low resistance
- Semi-noble, resistant to oxide formation, immune to fretting corrosion
- Self-healing

Ed Bock, senior consultant at APEX Electrical Interconnection Consultants, has more than 44 years experience in the connector industry (33 with AMP) in the areas of contact physics, electrical contact phenomenon, fretting corrosion, contact lubrication, and wear studies (tribology). His experience includes the areas of electric contact behavior and contact performance at both signal and power levels while subjected to various environmental conditions. He has studied the performance of various contact finishes and their respective failure modes and mechanisms and recommended countermeasures for improved reliability.