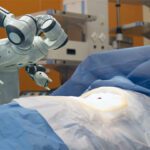
Silicone Alternative Offers New Option for Medical Cable Jacketing
Innovative component solutions that integrate cutting-edge technology with next-generation materials give medical devices designers new choices.
By Northwire (A LEMO Group Company)
Silicone is a popular cable jacketing material for components used in applications where reliable high performance is imperative. Commonly used in medical tools, hospital equipment, and surgical devices, silicone is flexible and smooth while still providing durability and thermal stability. When produced for medical-grade products, these qualities allow the material to meet FDA, USP Class VI, and ISO 10993 biocompatibility requirements and stand up to the intense demands of medical environments, including sterilization.
While medical-grade silicone is one of today’s most prevalent materials for healthcare device components, it still has its downsides. Producing sturdy, medical-grade silicone can be a long and costly process. As a thermoset material, silicone must go through a curing stage. This adds significant cost and a longer lead time to production. In addition, Siloxane, a key ingredient in silicone, experiences periodic shortages, adding lead time.
Silicone also has limited durability. The fast-paced environment of a hospital or clinic, with repetitive use, constant movement, and heavy rolling equipment, creates a harsh setting for cable systems that ultimately decreases the lifetime of the products. While the material resists medical sterilization, it is vulnerable to cutting, abrasion, and tearing. There is a need for silicone alternatives that retain the benefits of the material without the lengthy curing process, susceptibility to shortages, and physical weaknesses.
BioCompatic Cable Jacketing

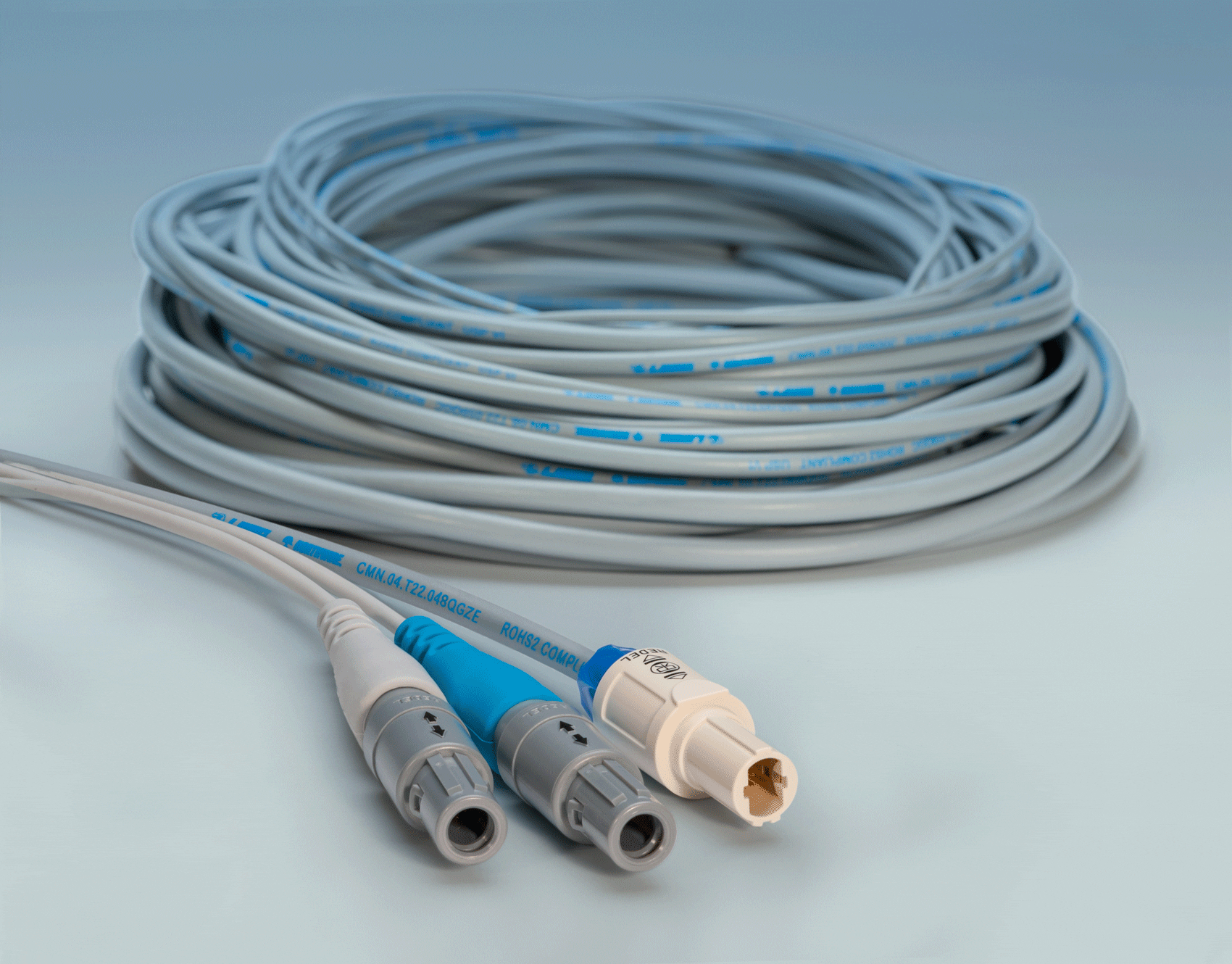
Northwire’s BioCompatic cable jacketing material (shown with LEMO’s Redel connectors) is compliant to USP Class VI, ISO 10993-5 & 10, RoHS3, and REACH. It is suitable for a wide range of medical applications, including patient monitoring cables, endoscopic reusable/sterilized assemblies, imaging cables, diagnostic tools, catheter applications, and other applications.
One USP Class VI medical cable jacketing option is a material called BioCompatic created by Northwire, a design engineering and custom cable manufacturer. This material offers biocompatibility and resilience yet is available at a lower cost than silicone with a shorter lead time. BioCompatic cable-jacket systems offer excellent endurance when subjected to steam autoclave and other sterilization procedures; are resistant to chemicals, abrasion, crush, and cutting; and are free of bisphenol, phthalate, and halogen.
Flexibility in action
Intense and demanding, medical procedures require tools that are not only biocompatible, but also ergonomic and user-friendly for health professionals. When a doctor applies constant motion or rotation to medical devices during a procedure, the cable powering or controlling that instrument is stressed. The repetitive torque and precession tangle or twist cable and can interfere with the operator.
To solve this problem, BioCompatic’s design allows for torque-free features. The retractable cable is engineered to optimize rotational motion, decrease resistance to repetitive flex, and maintain its original shape when not in use.
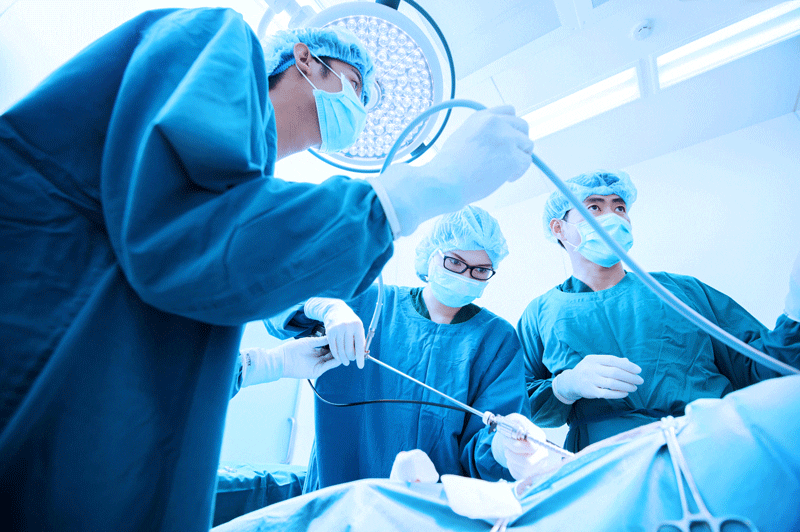
While BioCompatic retains the advantages of traditional silicone jacketing, such as biocompatibility and sterilization resistance, it is not limited to medical usage. The material’s robust strength, broad resilience, thermal stability, and ability to meet strict quality and safety standards lends it to a diverse range of extreme engineering applications in industrial, energy, aerospace, and defense industries.
Testing for endurance
Continuous and repetitive motion, like that exhibited in hospital or medical environments, greatly decreases the performance and lifetime of cable assemblies. When presenting a new material option to replace silicone and its well-known attributes, the cable manufacturer must perform ongoing, rigorous testing to verify cable flex life.
BioCompatic cables are subjected to an intense protocol of tests. A key metric was the silicone alternative’s chemical resistance to the most common hospital disinfectants and concentrates. Typically, medical cable and devices are not exposed to these substances for more than 10 minutes. Instead of only testing to realistic conditions, however, Northwire’s engineers immersed BioCompatic in these chemicals for a full 24 hours.
In addition to chemical resistance, BioCompatic was tested to withstand other factors common within hospital and industrial environments. Traditional silicone cable does not only face biocompatibility requirements; day-to-day use also takes its toll on the cable. A key example is gurney rollover. When a 200-pound hospital gurney rolls over a silicone cable assembly, tests show that the cable fails in fewer than 9,300 cycles. In the same tests, BioCompatic sustained rollover from the gurney in over 186,100 cycles.
Another example of typical usage comes with sterilization. For many applications, medical grade cable must undergo rigorous sterilization to avoid contamination by bacteria, viruses, and other pathogens.
A common way to sterilize a medical device or piece of equipment is to use a steam autoclave. The high pressure, high temperature steam effectively sanitizes hospital supplies, but can negatively affect a tool’s appearance and performance over its “cycle life.” BioCompatic demonstrated excellent resistance to 500+ autoclave cycles at 1340°C, including continuous operating temperatures of -800°C to 1050°C, as well as excellent resilience under gamma and ethylene oxide (ETO) sterilization, and H2O2 (STERRAD) sterilization.
Amid supply chain issues, material shortages, and other challenges to silicone-based electronic components, alternative material options for medical cables give OEMs an important tool to continue to innovate quality medical devices and equipment.
To learn more, visit Northwire online.
Like this article? Check out our other materials and standards articles, our Medical Market Page, and our 2021 Article Archives.
Subscribe to our weekly e-newsletters, follow us on LinkedIn, Twitter, and Facebook, and check out our eBook archives for more applicable, expert-informed connectivity content.