Battling Superbugs with Medical Equipment
Certain strains of bacteria are disrupting standard safety protocol in industries from agriculture and food preparation to medical equipment manufacturing and healthcare. The healthcare industry must reevaluate everything from patient care procedures down to the materials used in medical devices.
Strains of bacteria referred to as “superbugs” are becoming resistant to antibiotics.
Advancement and evolution are usually considered in a positive light – unless they occur in relation to dangerous bacteria. Termed “superbugs,” certain strains of bacteria are becoming resistant to antibiotics and disrupting standard safety protocol in industries from agriculture and food preparation to medical equipment manufacturing and healthcare.
“New data shows that far too many patients are getting infected with dangerous, drug-resistant bacteria in healthcare settings,” CDC Director Tom Frieden noted this year.[1]
Like the superbugs have done, hospitals also need to evolve and adapt to fight this threat. Practically, this means that the healthcare industry must reevaluate everything from patient care procedures down to the materials used in medical devices.
Looking Past Traditional Solutions
For decades, silicone has been the material of choice for jacketing medical devices, surgical cables, and other hospital equipment due to its ability to stand up to robust sterilization measures – a necessity with or without the risk of superbugs. The material itself offers many advantages, and silicone has benefited further from the development of anti-microbial coatings and similar additions. Nevertheless, even with its strengths, there are concerns that silicone is not evolving as fast as needed.
In recent years, there have been reports of dozens of infections and even deaths from medical scopes called duodenoscopes. Seven patients at the Ronald Reagan UCLA medical center contracted Carbapenem-resistant Enterobacteriaceae (CRE), one of the six most threatening superbugs, after endoscopies last year. Two of the patients died as a result. The scopes used were medical-grade silicone cables that had been sterilized; however, as the FDA noted in 2015, effective disinfection of the camera and cable assembly used in these procedures is difficult.[2] While the silicone material itself held up to stringent cleaning cycles, many experts questioned whether repeated rounds of sterilization – sometimes in the hundreds of cycles – is reasonable for today’s complex devices. Miniaturization, mobility, and moving parts in modern equipment may necessitate an upgrade in both materials and hospitals’ approaches to sterilization.
Exploring Single-Use Solutions
Single-use versions of fiber optic endoscopes and similar devices seem an obvious way to prevent any spread of disease. In a vacuum, this solution is ideal. In reality, the high cost of single-use medical equipment is too burdensome for most hospitals and healthcare facilities to implement.
“Cost is a huge factor when considering the workability of single-use medical devices,” says Kevin DePratter, the director of research and development at medical cable assembly manufacturer Northwire Inc. “It is a tempting plan, but rarely one that is feasible long-term. And, with better, more balanced options being developed, it’s an expensive and possibly disproportionate response to the superbug issue. This is where we really want to explore silicone alternatives that respond extremely well to repeat sterilization but are not intended to have such a long lifespan that over-sterilization or wear-and-tear that harbors bacteria is a concern.”
Viable Balance Through Innovative Solutions
Leaders in the medical cable assembly and device component markets see new thermoplastic elastomer (TPE) blends offering excellent advantages in limited-use applications. These materials typically handle 200 to 500 steam autoclave or disinfecting cycles. Compared to silicone-based components, which are often cleaned and reused close to 1,000 times, limited-use TPE blends control the life span of the product to allow for repeat use and cost efficiency without pushing performance or slipping into overuse.
In addition to striking the balance between long-term use and single-use products, TPE blends, like Northwire’s BioCompatic line, can offer benefits such as:
- Cost savings of up to 50% over silicone
- High resistance to crush, abrasion, chemicals, and other environmental factors
- Options for high flexibility, torque-free flex, and retractable cable features
- Adherence to RoHS2, REACH, USP Class VI, and ISO 10993-5 standards
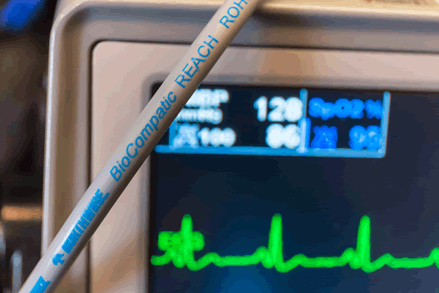
TPE blends, like Northwire’s BioCompatic, offer benefits and cost savings of up to 50% over silicone.
While it is unlikely that silicone will disappear from hospital device components altogether, it may be losing ground as the default material for medical manufacturers. The rise of superbugs has called into question the prudence of cable assemblies and other complex pieces of equipment that are cleaned and reused for hundreds of patients. Single-use equipment, a theoretically ideal choice, faces an industry that is often unwilling or unable to afford this option. By exploring limited-use applications with strong, durable, and low-cost silicone alternatives, medical manufacturers can contribute to a safer patient experience in a way that can be widely implemented in the not-distant future.
[1] “Superbugs Threaten Hospital Patients.” Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 03 Mar. 2016. Web. 27 June 2016.
[2] Qmed Staff. “Superbugs Could Mean Medical Cable Assembly Changes | Qmed.” Superbugs Could Mean Medical Cable Assembly Changes. Qmed, 12 Jan. 2016. Web. 27 June 2016.
This article was contributed by Northwire.



