How to Choose a Medical Connector Supplier
When you specify a connector type, you also choose a supplier. Beyond the connector’s design and functionality, there are a number of factors to consider when choosing a connector manufacturer for medical systems.
Many connector companies are very successful, generating continuous growth in revenue and earnings over many years, but it takes even more to succeed when serving medical markets. Let’s look at the key considerations when choosing a connector supplier for your medical designs.
The Right Management Attitude
The medical business is unique in many ways, requiring a different management mindset from other connector markets. Typical medical applications tend to be small-volume with lots of customization to satisfy the specific application. If product managers are accustomed to the high volumes and quick ramp commonly found in consumer or data markets, it is a major adjustment to deal with a high-mix, low-volume market opportunity.
Patience is also required from the financiers because it is common to have to tool a product years before significant revenue will develop. Medical products frequently require long qualification cycles followed by the typical slow ramp schedule as end users try the product in a limited way before finally committing to volume deployment of the technology. For the connector company, this means fronting the tooling investment, testing, and qualification, knowing that positive cash flow will be well down the road. Long development cycles inevitably also mean additional design refinements and modifications before production begins. Be sure the supplier you choose understands this development cycle and is prepared to comply with it.
Highly Talented Application Engineers
Because of the highly customized nature of medical products, the up-front specification and design process is very important. Each cable assembly must be custom-designed to meet the requirements of the specific customer, including performance, ergonomics, and aesthetics. Careful attention is needed in specifying the right acceptance criteria and testing required for qualification. The more highly skilled the up-front interface person is, the more successful the connector company will be. Traditional sales people, customer service, and distribution take a back seat in this marketplace.
Superior Mating Contact Systems
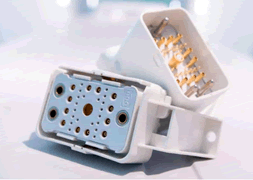
The ODU-MAC interconnect system offers 100,000 mating cycles.
The most successful medical connector suppliers all have a proven, high-performance contact design that can be used and reused in many different connector formats. Companies like ODU, for example, have a family of high-reliability contacts that are qualified for very high cycle life and can be snapped into a variety of housing configurations to meet the needs of a specific application. By having a compatible family of contacts with options for high power, small size, solder cup, crimp-to-wire, or press-fit, a manufacturer is able to satisfy a wide range of specific requirements with common contacts and custom housings.
Attention to Ergonomics

Fischer Connectors is famous for push-pull circular cables.
Medical connectors, especially cable connectors and assemblies, tend to be mated and unmated under the worst possible conditions. For example, monitor leads are often connected by people with little or no mechanical aptitude or experience. They are medical professionals and caregivers, not engineers. Therefore, the interface needs to be so intuitive that it is obvious how the cables are connected. Cables need to be robust and provide latching systems that are simple and highly reliable. For this reason, it is common to find push-pull or quarter-turn latching designs that are easy to operate. This becomes even more important when the person mating the cables has gloved hands. Successful medical connector and cable suppliers do a lot of up-front work to “goof-proof” a design so that it is so easy to use the operators don’t have to think about it.
This also applies to orientation and keying systems that ensure the connector is always mateable to the correct connector and never mateable to an incorrect one.
Cost-effective, Small-volume, Unique Solutions
Medical equipment suppliers usually require a cable or connector that will only mate to their own devices. This locks the end user into buying all the cables from the system manufacturer, ensuring quality, protecting warranties, and enabling traceability in case of a quality or performance issue. From the connector supplier perspective, it requires the ability to quickly and cost-effectively make a connector with unique features for a specific customer. Equipment suppliers also want to “brand” their equipment with a specific look and ergonomic feel. These important considerations require that successful medical connector manufacturers offer specific colors and surface finishes to meet detailed customer specifications. Power supplies and cable plugs need to be adapted for the country where the equipment will be used.
Deep Expertise in Materials
Medical applications have unique requirements including sterilizability, resistance to various fluids, temperature exposure, etc., that go well beyond the normal MSDS sheets provided by materials suppliers. Combine that with very specific OEM requirements for texture, lubricity, color, and flexibility, and you see that a connector manufacturer needs superior capabilities to identify candidate materials and processes, qualify them, validate that the material performs in the medical environment and meets all FDA specs, and can be consistently produced. This is not trivial, especially when combined with the small-batch nature of medical cables and boxes.
More than other industries, it is important that cable assemblies are flexible, feel good to the touch, and stay clean and hygienic in use. A number of materials have been developed specifically to achieve these attributes. This is especially true of cable outer-jacket and strain-relief overmolding material.
For this reason, OEMs and cable assembly houses frequently establish partnerships and exclusive relationships with materials and cable suppliers to guarantee continuous delivery of material with the right characteristics.

Ultrasound Comfort+ Probe from TE Connectivity
Procurement and QC organizations at medical connector vendors become a very important part of success for the company as a whole. Procurement people must find the right balance between having only a single source of material (more expensive, vulnerable supply reliability) and meeting all the consistency specifications needed.
Molex and TE Connectivity have both acquired multiple technologies such as flex circuit, flat-panel switches, sensors, fibers, and internal cable manufacturing to assemble a medical division to address the medical market. They also design and make higher-level assemblies that combine multiple processes to offer a complete end product.
Exceptional Process Engineering and Control

Lemo B Connector Family of push-pull circular connectors
Medical cables, in particular, require expertise in all aspects of the manufacturing processes. The processes involve steps like crimping, soldering, welding, and overmolding. These are processes that are difficult to automate and require exceptional skill, discipline, and dexterity to achieve error-free results. Before overmolding or sealing the assemblies, it is important that quality be thoroughly checked and performance measured to ensure high quality and yields for finished assemblies. The most successful medical connector and cable companies have mastered the concepts of CANBAN, lean manufacturing, TQM (total quality management,) and DFM (design for manufacturability) and have the leadership and dedication to make this second nature. The challenge becomes even greater as geometries shrink with ever-smaller, more-precise cables and connectors with higher-density interconnects in less space. The connector industry has learned the hard way that it is impossible to “inspect in quality.” The process needs to be well designed to ensure top-quality results with high yields.
Clean Room Manufacturing
Often medical connectors and cable assemblies must be certified as contaminant-free, and may be sterilized with heat or chemicals prior to placing in sterile packaging. Rigorous documentation, record keeping, and lot control is required so that any problem in the field can be quickly tracked to the source and contained. Clean rooms are not cheap, and personnel must be especially well motivated and trained so that discipline is maintained.
You will find that companies who have all of the attributes described above are likely to be decades old, with the ability to use standard contacts in a variety of shells and configurations to meet specific customer needs. They are attentive to the unique requirements of medical customers and have a very flexible manufacturing model to be able to make small batches of very-high-quality products with short lead times. A lot of these companies use screw machine-contact designs that are well suited to small batches. They use contact designs and plating systems suitable for more than 10,000 mating cycles. They also will have quick-turn prototyping capabilities that will enable them to make first pieces of high quality and full functionality to be able to support OEM first builds for their qualification and demonstration systems needed to win the end customers.
Medical connector and cable companies do tend to be sourced in the country where the OEM equipment is made. This has been a rich market for specialized connector companies in Europe and Japan as well as the US. Attention to high quality, consistency, and dependable delivery are all more important than price to medical OEM customers.
[hr]




