Design Considerations: Connectors for Compact Medical Devices
Compact device size is an overwhelming trend in modern electronics, but connectors for compact medical devices must be safe, easy to terminate, and widely configurable, in addition to small in size and extremely reliable.
James G. Dunbar, Product Marketing Manager – PCB Connectors, Phoenix Contact USA
 Innovations are critical in the medical field, and new devices are showing up on the market every day. It has been estimated that medical device makers spend up to 7% of their revenue on research and development, which is a much higher percentage than other industries. New wearable devices, such as glucose monitors and fitness monitors, are entering the market at a fast pace. Additionally, demand is rising for minimally invasive surgical procedures, which is driving the need for new handheld devices. Of course, in the medical field, safety is a primary concern. Connectors used in medical devices must be easy to terminate, as well as safe and reliable in the field.
Innovations are critical in the medical field, and new devices are showing up on the market every day. It has been estimated that medical device makers spend up to 7% of their revenue on research and development, which is a much higher percentage than other industries. New wearable devices, such as glucose monitors and fitness monitors, are entering the market at a fast pace. Additionally, demand is rising for minimally invasive surgical procedures, which is driving the need for new handheld devices. Of course, in the medical field, safety is a primary concern. Connectors used in medical devices must be easy to terminate, as well as safe and reliable in the field.
As with almost any design, the more options a connector manufacturer can provide for an engineer, the more flexible an engineer can be in their design. Since medical device designs are actively responding to market demands for smaller physical dimensions, smaller-sized connectors are widely needed. Products with a centerline spacing of 2.5mm provide a good compromise between size and flexibility in medical designs, can be obtained in one to eight positions, and, with UL-rated currents up to 5A at 150V, can easily power handheld devices used for surgical procedures. These types of connectors can generally handle 26 to 20AWG solid or stranded wires, which are large enough to handle 5A, but still small enough to enable the desired maneuverability of such handheld devices, providing device designers with much-needed flexibility.
Another consideration for medical equipment engineers is the configurability of a connector. It is especially helpful when all possible combinations are available, meaning: wire-to-wire, wire-to-board, board-to-board, direct wire to connector on the board (i.e., non-separable interface), and through-board connections. Permanent (e.g., board-to-board) connections are often utilized within medical devices, but through-board connections can also be desirable in especially space-constrained applications. External connections on these devices are typically made with more flexible connections, like wire-to-wire or wire-to-board, and are generally utilized to connect handheld devices, like probes, to primary equipment, like ultrasound machines.
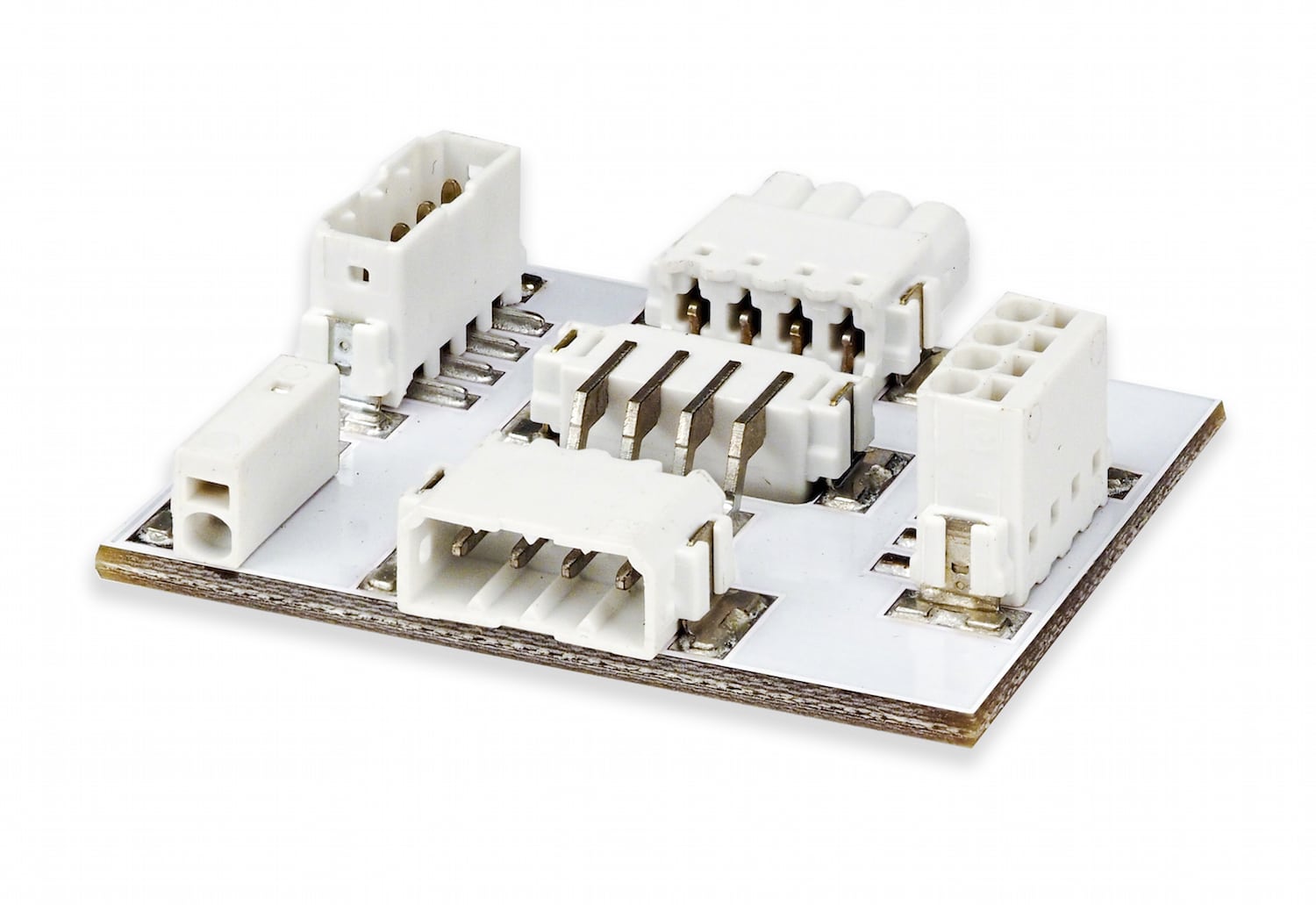 Figure 1: Connectors with multiple configurations enhance design flexibility for engineers.
Figure 1: Connectors with multiple configurations enhance design flexibility for engineers.
Finally, it is also critical to ensure that reliable connections are achieved when connecting such handheld devices to medical equipment. Ideally, the mating halves of a connector would issue an audible “click” to verify that the connector is fully mated. For even more secure connections, connectors with latches are available as well. Latches provide additional security against a connector interface accidentally becoming disengaged during a critical medical procedure, and should provide users with clear, tactile verification of proper mating.
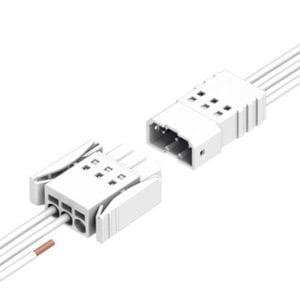 Figure 2: Latches easily ensure robust connections in high-reliability, space-critical applications.
Figure 2: Latches easily ensure robust connections in high-reliability, space-critical applications.
Medical devices will continue to evolve and become more complex as their enabling technologies continue to advance and, as they do, design engineers will continue to be challenged to achieve even greater functionality out of ever-smaller equipment in a smaller space. To help make their job easier, connector suppliers will continue to innovate, providing design engineers with an extended array of high-performance connector solutions with small centerlines and a wide range of connection methods.