Connector Design Considerations for Mobile and Patient-Worn Medical Devices
Medical device designers face some of the biggest challenges in the electronic design industry and some of the most stringent requirements in the world. To both assure safety and hasten time to market, it is paramount for designers to proactively reduce any and all risks associated with medical device designs.
Connector Designs for Medical Devices
Every medical device must satisfy a different set of parameters because every specification is based on how the device will be used, who will be using it, and how long it will be used. These types of usability questions must be answered early in the design cycle to avoid costly redesigns and trial delays. Medical device design teams need to know whether trained medical professionals will be operating the device or whether patients are the end users, as well as whether it is meant to be disposed of after a few uses or is expected to last for years. They also need to know whether it will be used in a dedicated clinical or hospital setting or carried from place to place.
In addition to ensuring that a device is low-risk, reliable, and functions to the highest expectations of a diverse base of medical professionals and their patients, manufacturers must meet yet another set of design challenges. To effectively compete in the market, medical devices must look modern, provide a positive patient experience, integrate more technology and functionality into ever-smaller physical packages, and work in an increasingly global market.
Connectors and cable assemblies play an important role in all of these trends, as they deliver power to hand tools and handheld diagnostic equipment and return signals to mobile consoles and surgical systems. They also work with sensors to keep warming blankets at the right temperatures and can even be part of vests, jackets, and other clinical clothing that can measure and help maintain a person’s health. Selecting the right connector and cable assembly solution can help achieve small, safe, stylish, and functional devices that hit the right price point to keep manufacturers competitive while minimizing risk for both patients and operators.

Medical connectors continue to evolve, offering new solutions designed to help manufacturers develop the next generation of mobile and wearable devices, including therapeutic exoskeletons.
Meeting FDA Requirements for Class I and Class II Medical Devices
Class I medical devices are low-risk applications, like external diagnostic devices and controls, and require very little in the way of government regulation to make it to market. Class II devices, like electric wheelchairs and infusion devices, carry moderate risk and therefore require more oversight to ensure they’re as safe and effective as consumers expect them to be. Class III medical devices (i.e., implantables) are subject to significantly more stringent FDA rules and oversight than the already rigorous approval process for Class I and II devices, and will thus not be addressed here.
General Connector Considerations
With some medical devices, unexpected failure can be a nuisance and an extra expense. With others, it can be the difference between life and death. So, it is important for medical device designers to know how many mating cycles a connector will be expected to last, whether an untrained patient or a trained medical professional will be operating and sterilizing it, and what kinds of conditions the device will need to endure. The answers to questions like these will affect details including material choices, sealing levels, and mating mechanisms.
Considerations for Wearable, Patient-Worn Connectors
In addition to sterilization, electromagnetic interference (EMI) protection, and ingress protection (IP) ratings, portable medical device designers should consider the following connector characteristics to ensure optimal performance.
- Connector Size — Take a close look at who will be managing the connection. A patient who is sick and self-managing will have different needs than a caregiver or medical professional. Make sure the connector is small enough to satisfy design requirements, but large enough for anyone to get a good grip on it. In addition, keep an eye on the profile of the connector. When it comes to wearables, the lower the profile, the better.
- Connections for Patient Mobility — Look at how and how much the patient will be moving. Highly mobile devices will require a different set of design considerations than those designed for bedridden patients. New options for the most mobile patients include connectors with concentric rings that allow for freer cable movement and reduce the risk of damage.
- Connector Torque — Many connectors are purposely designed to be difficult to connect or disconnect. Make sure that the connector is easy enough to connect and disconnect when needed, but difficult to accidently disconnect.
- Shape and Locking Types — Circular push-pull connectors are perfect for wearables, as there are no little tabs to break off, rectangles to challenge your spatial awareness, or USB-type hassles when you can’t find the right way to place the connector into the receptacle no matter how many times you turn it over. Circular connectors can also offer quick-release/no-lock solutions or screw locks if they need to stay in place in high-vibration applications.
- Cable Management — Wearables and devices that move around a lot require pre-planning for both cable length and functional mobility, or how the cables will need to move with the person or device. Think about sewing cables into clothing to minimize a patent’s ability to damage or tangle them and make sure there is enough, but not too much, cable. You may even want to consider offering different lengths of cable. In addition, robotic devices and exoskeletons with embedded sensors require protected cable systems.
- EMI Shielding — The more technology in the area, the more you need EMI shielding to protect the system from the noise. But you can’t skip EMI protections just because a product will have in-home use. Medical devices are often critical, so you can’t afford to gamble on significantly reduced electrical interference based on home use alone.
- Color — The more connectors in a design, the more color-coding you want to consider. To minimize the number of colors needed, consider multi-use connectors capable of transmitting signal and power or air and signal. Connector materials can often be ordered in different colors and rings that match connector or cable colors are widely available as well.

The Fischer LP360™ connector is one example of a new connector technology that is specifically designed for wearables and mobile equipment. Its 360° mating allows for easy cable management with no kinks.

Whether industrial or medical, innovative technology solutions for connectivity are working to reduce the amount of cable needed for mobile applications.
The Patient Experience
The global market for wearable medical devices is projected to approach $10 billion by 2023, with wearable heart rate monitors and electrocardiographs projected to do especially well. The US Bureau of Labor Statistics has projected that home healthcare services will be the industry with the largest wage and salary employment growth between 2014 and 2024, with a compound annual growth rate (CAGR) of 4.8%. These estimates reflect the desire to put healthcare in the hands of patients and create additional pressure to ensure that interconnect solutions must perform perfectly, even in less-than-sterile conditions.
With more self-care comes a greater need for patient adherence, which is affected by the way a device is designed. A medical device for home use should be easy to use, easy to sterilize, and fit naturally into the patient’s existing routines. If it’s a wearable, it should be comfortable, and if it’s handheld, it should be easy to hold and carry.
Connector designs for these applications have their own specific challenges. Connectors should be easy to use for both the youngest and oldest patients. So, the torque required for mating and unmating should be carefully considered and similarly shaped connectors should be color-coded or otherwise clearly distinguished. Plastics offer a wide range of color-coding options, but color-coded aluminum materials are available as well, and you can also integrate colored rings in your design to make mating even easier for users. Connectors for wearable and handheld medical electronics should also be sturdy enough for minimally trained patients to use and clean without worry and rugged enough for long-term use. Disposable connectors are gaining in popularity and offer cost-effective solutions designed to be robust and reliable for single- or short-term use. Examining the full range of requirements early in the design cycle will help determine whether your device can take advantage of these and other new connector technologies.

The Fischer Disposable Series is comprised of cost-effective push-pull connectors that deliver high-quality performance, but are designed for disposal after a short number of uses. Whether directly mounted on a disposable hand piece or overmolded or mounted to a disposable cable, these plastic connectors offer multiple configurations to suit medical applications ranging from catheters to surgical hand tools.
Appearance Matters
Patient experience isn’t only about function. The look and feel of a device affects the overall experience, which, again, affects patient adherence. For instance, it has been shown that adolescents suffering from idiopathic scoliosis are more likely to wear an uncomfortable brace if it’s aesthetically pleasing and patients are less likely to continue taking a drug if it looks different than it used to, even if it’s medically necessary.
Miniaturization
Although a host of non-medical applications have quickly adopted the small, powerful connector solutions that have recently become available, medical device manufacturers are often slower to adopt the newest connector technology. There are several reasons for this.
The medical device market moves more slowly than consumer, military, and industrial markets due to extensive FDA trials and regulations. Medical device designers also often wait to see how connector solutions work in other markets before adopting them as low-risk alternatives to their existing solutions. In addition, the demand for miniaturized devices is often not be as urgent as in other markets, especially with regard to mobile surgical equipment and patient care applications where the patient experience necessitates a stronger focus on aesthetically pleasing equipment sets.
One application area where miniaturization is succeeding is in med-tech. The newest innovations that are currently revolutionizing healthcare and how it is delivered are successfully implementing designs that take advantage of a new class of rugged connectors that carry both signal and power. This trend is now expanding into some of the newest mobile devices, including patient-worn devices that patients monitor and manage and new devices that incorporate technology such as sensors that report back — sometimes wirelessly — to a base station.
Designing tiny connectors comes with another set of challenges. The choice of material, the number of pins, the size of each pin, and the amount of electricity each pin can handle will influence the creepage and air clearance requirements, which affect how many pins can fit in a smaller space. Higher pin densities and hybrid configurations allow a single small connector to do what would otherwise require several larger connectors, transmitting data and power together without interference.

The Fischer MiniMax™ Series of miniature, rugged, and ultra-high-density mixed power and signal connectors provides up to 75% weight savings and 45% space savings in medical and industrial application. They also deliver up to 10Gb/s data transfer and 5,000 mating cycles, are compatible with USB 3.0, HDMI and Ethernet protocols, offer three locking systems, and can be sealed to IP68.
Successful miniaturization of a connector solution not only depends on the connector design, but the cable as well. It is imperative to match connectors with correctly sized cables, which may involve customization to ensure proper fit and mitigate interference, and to thoroughly test connector and cable pairs.
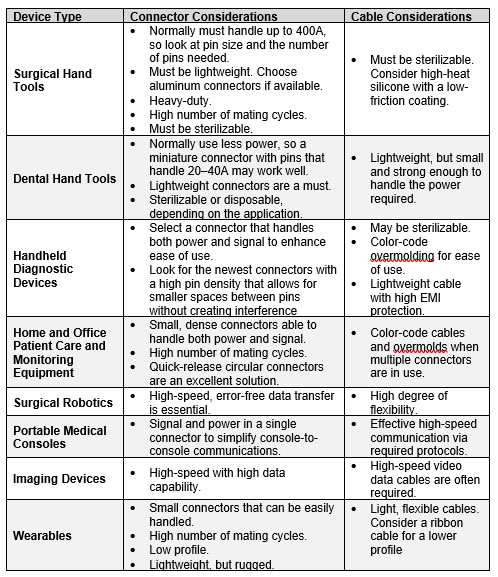
Miniaturization Application Examples
Complete Cable Assemblies
In any device, the cable — which may carry power, data, or both — is as critical as the connector itself. Unless a device uses disposable batteries, or until wireless charging technology is the norm, every device will need to be plugged into a power outlet to either operate or recharge the batteries. Alternately, cable needs for data transfer depend on the end use application. For some mobile and wearable devices, a wireless data transfer solution is desirable, as it gives users greater freedom of movement. However, a cable that uses protocols like USB, Ethernet, or HDMI would be more secure and would often provide faster and significantly more reliable data transfer than technologies like Wi-Fi or Bluetooth. So, when security, speed, and reliability are vital, designers must choose the best cable for the application.
Cable choice, like everything, is based on the end-use environment. A silicone overmold might be necessary if the cable will be subjected to high heat, like autoclave sterilization processes. A low-friction coating might also be helpful for preventing damage over the lifetime of the cable. Designers should also consider whether the application requires sealed cables that are safe to submerge in fluid, special EMI/RFI protection, and straight or right-angle overmolds. It is also imperative that designers ensure that each cable and connector pair are compatible with each other.
Ensuring Application Success
In addition to following the guidelines offered here, working with a trusted connector supplier experienced in medical device designs can help ensure patient and operator safety, as well as successful trials, and can hasten time to market — especially since each medical device must satisfy a different set of parameters depending on how the device will be used, who will be using it, and how long it will be used.
By Patrick Kinyanjui, Principal Engineer at Fischer Connectors, Inc.
Like this article? Check out our other 2019, Connector Basics, and Medical Market articles.