Medical Devices and Instruments Put the Squeeze on Connectors
Rapidly evolving medical technologies are increasing demand for small, lightweight, high-density connectors that can handle high signal speeds, endure frequent mating cycles and sterilization, and play well with sensors.
Connectors for electronics in medical devices, equipment, and instruments cover a very broad range of uses. They, and their accompanying cables, are also likely to be customized for specific applications in this market. The biggest trends in medical technology include ongoing demand for more sensors producing more data, higher signal speeds, and greater pin densities, all in a much-reduced space. Some drivers of these trends are new or improved medical devices such as portable prosthetics, wearable thin-film “skin,” and implantable or intra-body devices, as well as single-use devices and robotic remote surgical systems.
For example, a new prosthetic arm developed by researchers at the University of Utah senses touch and responds to its wearers’ thoughts. The LUKE arm, named for Luke Skywalker’s robotic hand in “The Empire Strikes Back,” sends biologically realistic signals to the brain using electrodes implanted in the wearer’s nerves, mimicking the way a real hand feels objects. Amputees can sense whether an object is soft or hard, understand how to pick it up without causing damage, and perform delicate tasks that standard prosthetics can’t do with their metal hooks or claws.
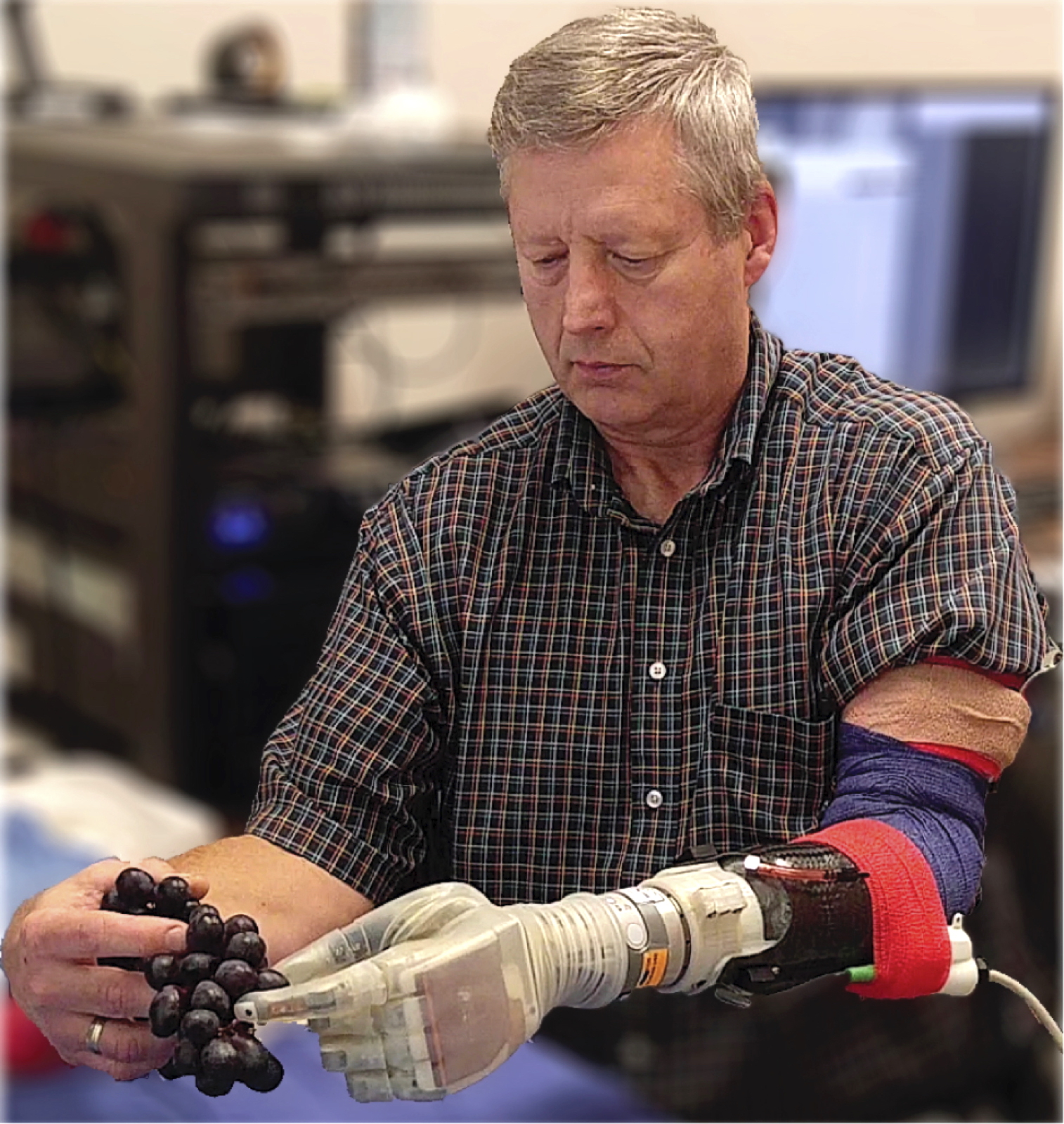
The LUKE prosthetic arm can pick grapes without crushing them or pick up an egg without cracking it. (Source: University of Utah Center for Neural Interfaces)
MIT researchers have developed an electronic pill that, when swallowed, delivers drugs or transmits diagnostic information using wireless Bluetooth technology. The capsule can stay in a patient’s stomach for up to a month, using sensors to detect vital signs, infections, or allergic reactions. One use case is patients taking chemotherapy or immunosuppressive drugs over long periods, who must be monitored for possible reactions. The capsule can also be designed to communicate with other nearby wearable and implantable medical devices, to pool and transmit data to a doctor’s or user’s smartphone.
An ingestible sensor can lodge in the stomach for a few weeks to deliver drugs or detect vital signs, and communicates wirelessly with an external device. (Source: MIT)
Wearable electronics are so thin that one R&D project can also sub as robotic “skin” for prosthetics. University of Houston researchers developed an ultra-thin and stretchy wearable electronic device that wearers barely notice. It automatically collects data and sends it back to the wearer and can perform multiple functions, such as sensing, switching, stimulation, and data storage.

A soft, stretchy data collection material can be used as a wearable device on human hands, or as robotic “skin” for prosthetics. (Source: University of Houston)
Connector Needs
As in industrial and some military and aerospace markets, the general mechanical connector needs of electronics in medical devices and equipment are light weight, ruggedness, high density, and high shock resistance. The medical market is especially multifaceted, with separate needs for patient use, prosthetics, wearable sensor applications, doctors’ office detection and monitoring, surgery, operating room EMI management, implantables, and remote and robotic surgery, said Bob Stanton, director of technology for Omnetics. Other considerations include feel, appearance, cable flex, and materials that don’t harbor biological growth.
As density and signal speeds increase, Omnetics is seeing rapid changes in connector-to-cable systems with electronic elements that demand much lower voltage and current levels. “We’re seeing millivolt systems in items from pulse oximetry for monitoring a person’s oxygen saturation levels to skin sensors, and even EKG-capable wristwatches,” said Stanton.
Omnetics’ plastic micro-circular (0.050” pitch) connectors are being used to customize medical devices, since they are small, lightweight, and readily available. (Source: Omnetics)
In portable prosthetics, connectors must be smooth to the touch, easily re-connected, and cleanable in-home. Ultra-miniature connectors for motors or detectors in doctors’ offices that can be immersion-cleaned must be sealed and waterproof. Connector and cable systems for multi-spectral high-speed imaging are changing the oscilloscope industry from top to bottom. And medical neurology systems in the surgery room depend on very low electrical resistance and total isolation from outside signals.
Omnetics offers 0.050”- and 0.025”-pitch micro- and nano-connectors designed for the higher-speed digital signals used in today’s medical devices, said Stanton. Low dielectric-constant materials that support higher speeds and fulfill the application’s strength and bio-compatibility requirements are required. In addition, both micro- and nano-sized contacts can be included within one connector when this gives the device an advantage.
Applications for ODU’s range of medical connectors include MRI machines, inhalation devices for respiratory therapy, dental care treatment units, mobile X-ray devices, clinic communication systems, fetal heart rate monitors, handheld pulse oximeters, and intraoral camera systems.
One new product from ODU for medical and other applications is the hybrid ODU-MAC PUSH-LOCK connector, part of the company’s ODU-MAC Blue-Line portfolio. These are modular, easy-to-use manual mating connectors that make it easy to combine many individual connectors into one. The new PUSH-LOCK is even more compact, reflecting the growing miniaturization trend, and can be custom-fitted with up to 70 signal contacts and power, coax, air, fluid, and data rates. It can withstand up to 5,000 mating cycles, is IP67 compliant, easily cleanable, and has a push-pull design for secure connections and space savings. The connector can also be operated with just one hand.

Reflecting the growing miniaturization trend, the compact ODU-MAC PUSH-LOCK can be custom-fitted with up to 70 signal contacts. (Source: ODU)
In addition, ODU’s new hermetically sealed MINI-SNAP circular connector has push-pull locking and a robust metal housing ideal for medical applications. It’s capable of up to 5,000 mating cycles and up to 500 autoclaving cycles. Once mated, the ODU MINI-SNAP locks itself into the receptacle, ensuring a reliable connection for power, signal, or data.
LEMO offers a wide range of plastic medical connectors under its REDEL brand. The plastic materials used in these connectors — including polysulfone (PSU), polyethersulfone (PES), and other PSU-based materials — are FDA approved in their respective natural colors. Applications include helping ensure reliable connections on analyzers and processing equipment for data gathering, dental equipment, electrosurgical devices, disposable devices, pacemakers, and hearing devices.
Requirements and Standards
Connector materials used for medical-grade devices are well specified to ensure low risk to the patient and good service to the practitioner, said Omnetics’ Stanton. When it comes to materials used in direct patient service, the surface and tactical feel of the connector and cable are always important. Many materials are application specific. For example, in addition to being small and rugged, most medical connectors must be made of materials that can be cleaned by green soap, immersion, ethylene oxide (EtO) sterilization, or autoclave. These specifications are covered in the ANSI/AAMI EC53 standard.
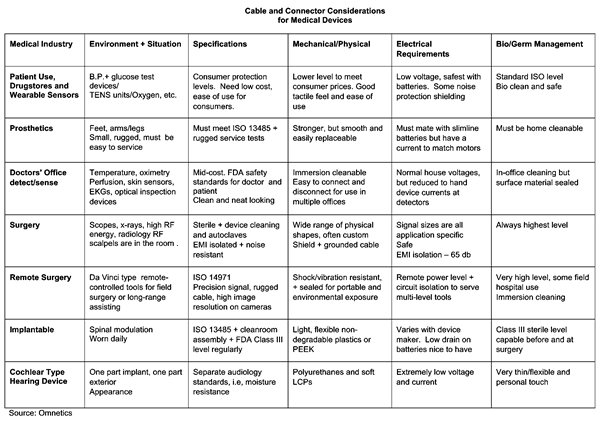
Medical Connectors Table (Source: Omnetics)
The standards most often used for medical cable and connectors are IP67 or IP68 and ISO quality standards, said Stanton. ISO 13485 is a quality management system that connector suppliers must follow to ensure they are planning, acting, and reporting their processes consistently to maintain continuous quality and error-free products.
For marketing in the US, device manufacturers follow complete FDA standards and specify which requirements affect cables and connectors. The FDA uses a three-class rating system for most medical instruments and devices and their components. Electrical and electromechanical devices that must be supported with connectors and cables, and that fit into these classes, include such disparate items as spinal modulation instruments, catheters, perfusion monitors, brain mapping tools, and cochlear implants.
Understanding how a product will be classified is complicated and often falls to the OEM submitting the product to the FDA, said Stanton. “Often, new devices are quite unique and Omnetics is invested in meeting the level specified by the device manufacturer,” he said. In addition, a number of other sustainability and performance details are needed.
Some new and upcoming developments in medical devices and equipment will demand new or different types of connectors, said Stanton. For example, visual imaging systems used in surgical procedures will require very high-speed data sensors that send images from invasive probes and scopes to the examining physician. A new family of differential-signal-pair cable and connector sets are currently being used for this purpose. Also, ultra-thin sensor chips, bio-detectors, and MEMS chip technology are changing the needs for some applications. Precision electronics will make these developments and others possible.
Like this article? Check out our other Connector Basics and New Technology articles, our Medical Market Page, and our 2019 Article Archive.
- Underwater Connectors Get Smaller and Tougher for Submarines and UUVs - November 10, 2020
- Small Military UAVs Demand Smaller, Lighter Connectors - August 4, 2020
- Commercial Connectors Toughen Up for the Harshest Environments in the Universe - June 2, 2020





