Implantable Medical Devices Depend on High Quality Connectors
The cables and connectors used in medical devices are based on common design rules and similar products are used in other markets. However, they are tailored to the unique demands of medical applications and are heavily scrutinized throughout production.
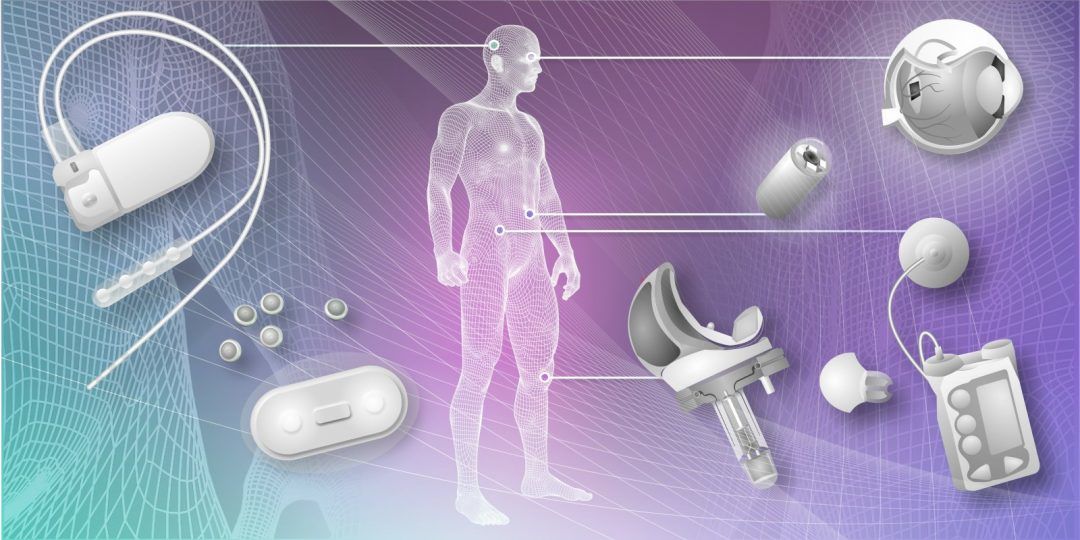
Understandably, safety and reliability are at the forefront of considerations for implantable medical devices. The cables and connectors used in these devices are based on common design rules and similar products are used in other markets. However, they are tailored to the unique demands of medical applications and heavily scrutinized throughout production. Everything from the materials used to the manufacturing of these components must adhere to strict controls and specifications.
Axon’ Cable makes cables and connectors for implantable medical devices and is equipped with an ISO Class 7 cleanroom to manufacture components. “With laminar flow we can go down to ISO 5 to guarantee a very low contamination level,” said Stephanie Achart, deputy engineering manager at Axon’ Cable. Laminar airflow helps reduce turbulence, thereby preventing the spread of contaminants.
Cleanrooms are used to manufacture components for industries where cleanliness is a priority. These enclosed areas are engineered for precise control, monitoring, and maintenance of the environment within. The International Organization of Standardization (ISO) provides cleanroom classifications based on international standards for acceptable cleanliness. They regulate temperature, humidity, airflow, filtration, and pressure. ISO classifications range from Class 1 (the cleanest) to Class 8 and are determined by the number and size of particles permitted per volume of air in a specific amount of time.

Axon’ Cable’s silicone overmolded assembly with one custom connector for use in surgery rooms. Similar assemblies are developed for implantable solutions using custom connectors with overmoldings. If assembly is transcutaneous, standard connectors can be also integrated outside the body.
Final compliance for the technical requirements or regulations needed to get the devices on the market is the result of cooperation between Axon’ and its customers. “Axon’ being ISO13485 certified is fully aware and qualified to supply any required documentation, and to guarantee the required level of quality and traceability,” Achart said.
Through this close collaboration, Axon’ has gained a level of expertise with the materials. “The materials are really restricted because of the long-term implantable requirements. Not many materials are available on the market for that. We know the manufacturers of those materials very well, and we know how to use them,” said Archart. Axon’s R&D department evaluates the performance of the materials regarding adherence, for instance to meet overmolding requirements.
Similarly, the conductors or any metallic parts that are used must be resistant to body fluids. The sealing must be perfect to make sure those fluids don’t come in contact with the metal parts in the connector. This is important to avoid any shock or current leakage, but also to prevent any possibility of contamination trapped anywhere inside the device before it is implanted.
“In addition, such development projects require that everything has very good traceability. At some point we will freeze the design and then nothing can be changed. No supplier, nothing in the workflow can be changed without discussion or even sometimes requalification. There is a lot of exchange with the customer at this stage of development,” Achart said.
Another step that Axon’ takes to guarantee the non-contamination of the assemblies is to perform some control of the bio contamination at various stages. “This involves, for example, taking bacteria cultures from the gloves of the operator and on the table where they are working. In our own internal lab, we count the colony-forming units (CFUs) to evaluate the contamination and make sure it is not too high. We also use an external lab to check our control, to make sure the way we count is good enough.”
These standards do not only apply for devices that will go into humans. Axon’ also makes products to be used in research, for implantation into pigs and mice. One such development project aimed to electrically connect fibers in the spinal cord to stimulate regrowth of nerves that were severed due to an accident or injury. Another product, the assembly that goes between the pump that is fixed on the heart and the control unit outside the body, is for the development of a ventricular assistance device (VAD). A similar project provides the assembly that goes from an artificial heart to an external control unit.

Axon’ Cable’s assembly developed for research in spinal cord repair. The left connector is sewn on the pig’s skin and right connector is fixed to the spine. The cable is extensible to allow easy movement of the pig’s abdomen.
“For the cable, the biggest specificity of implanted devices is the use of very specific conductors that can still be insulated with an extrusion process in a standard way. Then wires can be assembled and extruded again, or molded,” Archat said. This is done at Axon’s facility in Montmirail, France. “For the connectors, we subcontract machined titanium or stainless steel housing and assemble them with some specific welding processes and load the contact inside. We install them and we make the potting material. Everything is specifically adapted to the customer’s system. Each connector is suited to the artificial heart or the pump because it needs to be really integrated into that design.”
Tiny, implantable connectors
A connector for a pacemaker or other implantable medical device may be as tiny as 1 mm pitch. Clément Bidault, product development engineer at PRECI-DIP, explained that for connectors of this size, screw machining and Swiss quality come together to greatly enhance the product. Screw machining uses spinning lathes to shave down metal to the desired size and shape. “In this way, we can have tight tolerances on those brass contacts. This is how we can add such a small contact onto such a small connector, while ensuring that the product conforms to the customer’s specifications,” he added.

PRECI-DIP designs and manufactures connectors for life-or-death performance-linked products, including contacts and miniature connectors used to connect printed circuit boards in pacemakers and defibrillators.
The real advantage of screw machining is that the external shape can easily be customized and it doesn’t cost a lot to make a new tool, said Bidault. At PRECI-DIP, the body of the contact is made in this way, then a clip is inserted inside the body. The clip is stamped, but it is a standard part. “We have about 50 clips with different sizes. We always use the same clip so we can customize the contact to meet every customer requirement with this technology, even for low volume applications.”
After the screw machining comes the plating. Most applications use gold plating, but PRECI-DIP also does more advanced plating with rhodium or palladium. “We have a lot of technology to fight corrosion,” Bidault said. This is particularly important for implantable medical devices because they must be highly resistant to blood and other bodily fluids.

The Restriction of Hazardous Substances (RoHS) lead-free directive now incorporates medical electronics. Even if there is an exemption for machined contact with brass, PRECI-DIP already proposes lead-free interconnect solutions to its customers. PRECI-DIP designs and machines miniature socket contacts for hearing aids. Those contacts are made of a lead-free brass barrel and an elastic clip, plated nickel free as they would be in contact with the patient skin, and assembled automatically assuring 100% the integrity of the assembly.
PRECI-DIP follows the customer requirements and then institutes its own controls to ensure the quality and reliability of the connector. Bidault explained that following the customer’s specifications, using the right plastic housing material, for example, is easy to do. What is difficult is consistently delivering the high level of quality that is required. For this, a specific assembly machine is created for each project. PRECI-DIP uses cameras to monitor production to catch a missing or broken clip, or other issue. This type of control is an automatic check done by a robot, which is highly reliable and offers greater performance at a reasonable cost.
Production of medical devices is a complex process, compared to industrial production. Each machine has one robot arm used for assembly and another for quality control. The tiny connectors require very fine work by the robots. “Sometimes we have to assemble the contacts one by one because they are so tiny. PRECI-DIP’s automatic assembly technologies are quite advanced,” said Bidault.
To learn more about the companies mentioned in this article, visit the Preferred Supplier pages for Axon’ Cable and PRECI-DIP.
Like this article? Check out our other technology trends articles, our Medical Market Page, and our 2023 and 2022 Article Archive.
Subscribe to our weekly e-newsletters, follow us on LinkedIn, Twitter, and Facebook, and check out our eBook archives for more applicable, expert-informed connectivity content.
- Sealing Success: Overmolding for More Secure Connections - April 23, 2024
- Medical Cable Assemblies Product Roundup - April 23, 2024
- Mezzanine Connectors Product Roundup - April 16, 2024