Six Specification Considerations for Medical Connectivity Components
As medical equipment continues to scale down in size and up in connectivity and capabilities, new materials, device designs, and industry standards combine to create innovative medical connectivity components that satisfy rigorous form, fit, safety, and performance requirements.
By John Koon and Connector Supplier medical connectivity components
As medical equipment continues to scale down in size and up in connectivity and capabilities, new materials, device designs, and industry standards combine to create innovative medical connectivity components that satisfy rigorous form, fit, safety, and performance requirements. To ensure optimal selections, achieve peak performance, and safeguard against failures in high-stakes medical applications, designers should consider six core characteristics when specifying medical connectivity components like connectors and cable assemblies: safety and reliability, durability, materials, form factor, performance, and next-generation innovations, including space-saving hybrid power and signal solutions and single-use, disposable technologies.
Safety and Reliability
Connectors and cable assemblies used in hospitals, outpatient facilities, and home health care equipment must meet rigorous industry standards, such as IEC 60601, as well as environmental sealing and material safety standards, to ensure the reliable operation of the product and the safety of both patients and operators. For example, the plastics used for cable jackets and connector housings must be proven safe for use in hazardous medical environments, and especially so if they’ll be subjected to prolonged patient exposure or sterilization processes including autoclaving, ionizing gamma radiation, ethylene gas, and STERRAD plasma. In addition, connectivity components employed in MRI machines must not employ any magnetic materials and high-power and high-current connectors and cables must employ touch-proof designs that prevent patients and operators from accidental contact and protect them from electrical shock.

Cinch Connectivity Solutions’ Johnson line of non-magnetic coaxial connectors for magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and other medical applications are made from high purity copper alloys to ensure the absence of magnetic ferrous materials.
“In medical applications, safety is of the utmost importance. All connectors should be touch-proof and allow no electrical shock to take place — for patients or operators. That is why following safety standards like IEC 60601 is so important,” said Steven Lassen, senior customer application engineer at LEMO Connectors.
The critical importance of complying with medical safety and reliability requirements makes researching applicable industry standards a logical first step when specifying medical connectivity components, especially since redesigns tend to be costly and can significantly delay a product’s time to market.
Durability
Another factor to consider when selecting connectors and cable for medical device designs is durability. Medical connectivity components must be designed to reliably transmit power, data, and/or signals despite exposure to a variety of environmental hazards, including electromagnetic interference (EMI), fluids, gasses, extreme temperatures, and rough handling.
For example, medical devices equipped with cable assemblies that are plugged and unplugged frequently should specify connectors designed to withstand high mating cycles. Connectors and cables designed to endure shock and vibration without negatively affecting performance can also be useful in high-mating-cycle applications and are often mandated in mobile medical applications, which can range from handheld or wheeled patient monitoring devices to off-site imaging equipment, like mobile MRI and X-ray machines. Similarly, if a medical cable will be subject to frequent bending, it’s important to specify one designed to withstand bending, rolling, torsional, and variable forces without degrading the cable’s physical properties (e.g., fraying the cable jacket) or electrical performance.

For example, Fischer Connectors’ Fischer Core Series Plastic connectors (pictured above) provide user safety and electric shock protection over long lifetimes in medical, industrial, instrumentation, and food and pharmaceutical processing applications. The fully insulated plastic connectors are lightweight, sterilizable, corrosion-resistant, easy to use, and extremely durable. Features include plug bodies that support secure handling with gloved hands, color-coded plugs and receptacles that enable easy identification and prevent mismating, and a push-pull locking system that prevents accidental unmating, all potential issues in a busy hospital setting.
Materials
Medical connectivity components often require specialized materials designed to meet rigorous safety standards and reliably withstand environmental hazards including sterilization processes. Northwire, a LEMO Group company, manufactures a USP Class VI medical-grade polyphenylsulfone (PPSU) material called BioCompatic that it ideal for medical connectivity components. The material is an alternative to silicone and is highly resistant to chemicals, cuts, and abrasion. It is also designed to withstand steam, gas plasma (H2O2), gamma radiation, and ETO sterilization.
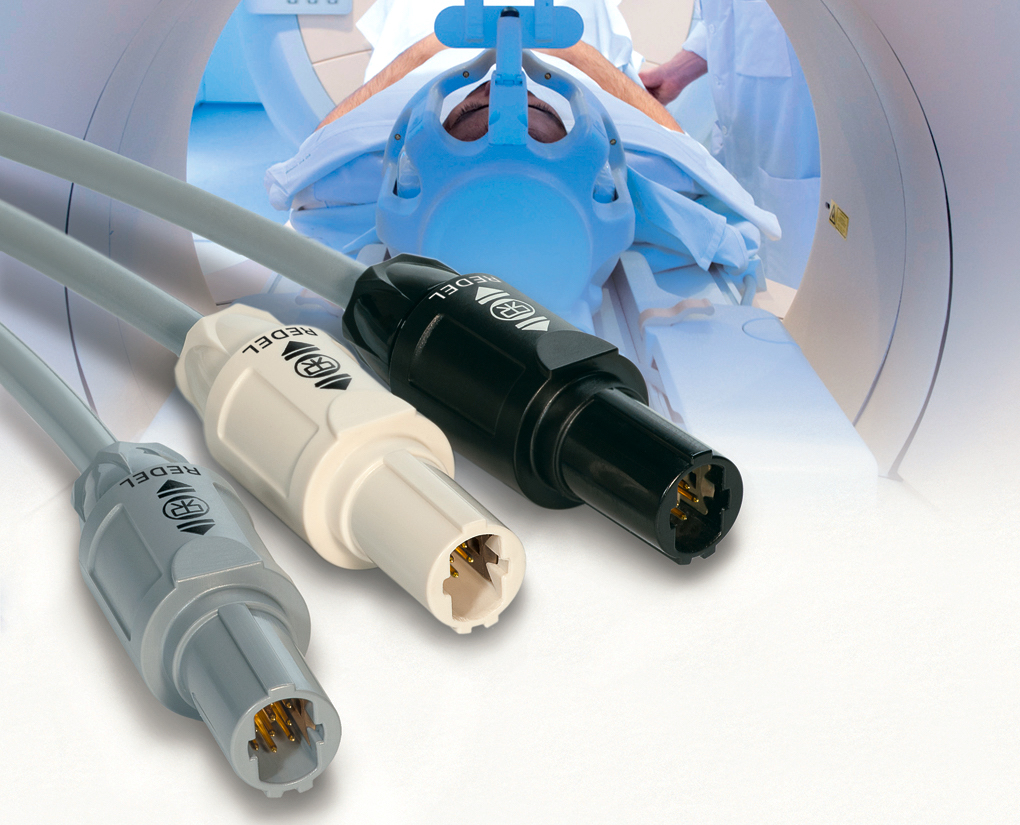
LEMO’s REDEL SP Series push-pull medical connectors features BioCompatic cable assemblies.
LEMO’s REDEL SP self-latching, plastic, push-pull connectors feature an ergonomic grip, several color-coding and keying options to help ensure proper mating, and rugged resistance to chemicals, shock, and sterilization processes. The series employs a rectangular insert for higher contact density and a recessed latch sleeve for greater resistance to shock.
Nicomatic has developed a lightweight shielding technology designed to accommodate dynamic flexing demands in applications that require high signal integrity. The shielding is applied to flat flexible cabling (FFC) during the manufacturing process and provides effective protection against EMI, radio frequency interference (RFI), and electrostatic discharge (ESD). In a medical setting with numerous machines and devices, proper shielding is essential to protecting the functionality of each machine.
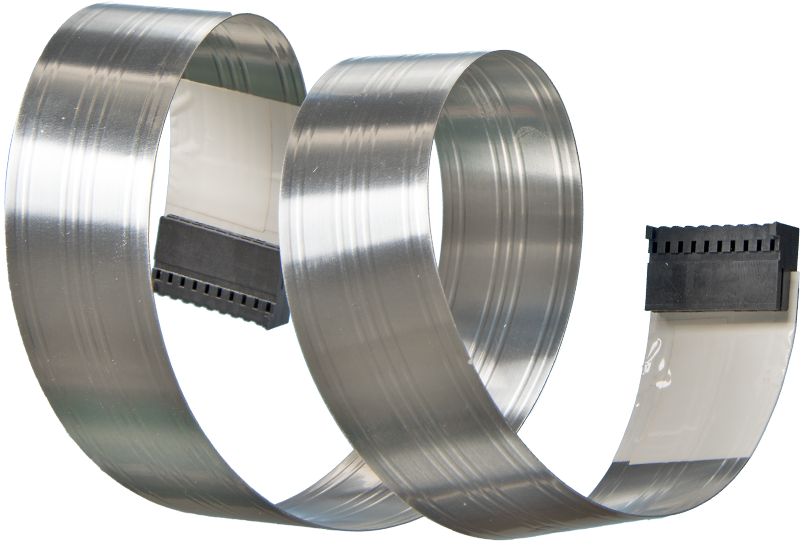
Nicomatic’s V-Shield technology for flat flexible cabling (FFC) provides superior EMI, RFI, and ESD signal protection.
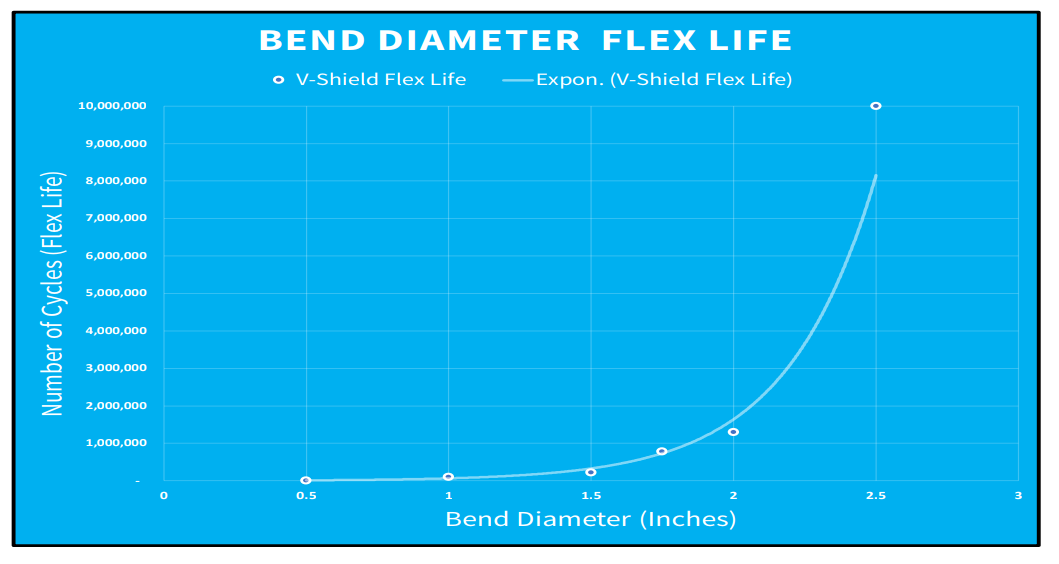
Durability testing for Nicomatic’s V-Shield technology demonstrates a flex life of 11,700 cycles with a bend diameter of 12.7mm (0.5”) and 10 million flex cycles at a bend diameter of 63.5mm (2.5”).
Another method used to protect the delicate electronics found within many medical cable assemblies is to coat them with a layer of Parylene. The trade name for a variety of chemical vapor deposited polymers, Parylene provides a moisture and dielectric barrier on metal, ceramic, and plastics that can be repeatedly sterilized without degrading the coating. An FDA-approved, USP Class VI polymer, Parylene conforms fully to substrate contours, crevices, and edges and the thin transparent film adds minimal weight or volume. Also, because of its transparence it can be used to coat optical devices. This is one of the many methods offered by Carlisle Medical Technologies, and is a common device coating for guidewires, needles, catheters, electronic circuitry, and molded products.
Form Factor
Even as connectors become smaller and lighter, high-quality contacts and secure locking features remain critical. Form and fit are important as well, and especially so when third-party cable assemblies are mated with device connectors. Terminal position assurance (TPA) devices, polarization features, keying mechanisms, and scoop-proof contact designs can all help ensure proper mating and protect contact integrity. In addition, thoughtfully designed form factors can also help hasten and ease installation and integration processes.

One example of a medical connectivity component with a form factor designed to anticipate and overcome a number of medical market demands is ITT Cannon’s QLC Solderless plug (pictured on the right). This 260-pin, zero-insertion-force (ZIF) connector has a dual-card-edge and slider design that eliminates the need for hand soldering fine-pitch contacts onto PCBs, enables simple, manual insertion, achieves stable, high-contact-force connections to PCB assemblies, and significantly reduces assembly process time and cost.
Performance
Medical connectivity components must employ high-quality, high-reliability contacts to provide secure, reliable connections. Long mating lifecycles, very low contact resistance, and low insertion and extraction forces are other important features for equipment that sees frequent use by multiple operators. Since contacts are the crucial core of all connector technologies, specifying medical connectors with ruggedized contact designs made using high-quality materials is critical to achieving high performance in harsh medical environments.
Smiths Interconnect’s Hypertac hyperboloid contact technology (pictured above) was designed to support high-reliability performance in hazardous environments, including medical applications. These contacts exhibit low insertion and extraction forces, self-cleaning capabilities, immunity to fretting corrosion, and 30% higher current carrying capacity than standard contacts of the same size.

Smiths Interconnect offers many medical connectivity components enabled with its Hypertac hyperboloid contact technology, including its HyperGrip Series user-keyable and -configurable circular, plastic, color-coded push-pull connectors. Designed to meet medical industry requirements, such as finger-proofing to UL544 and IEC 60601-1, the series features a front- or rear-panel-mount receptacle design that allows users to mount the harness assembly from the inside or outside of device enclosures.
Next-Generation Medical Connectivity Components
As more medical devices join the Internet of Things, communicating information across patient care teams, scores of innovative, next-generation medical connectivity components are being released to market, including customizable connectors and cable assemblies designed to meet unique application demands unmet by off-the-shelf solutions, hybrid connectors capable of combining data and power in a single, space-efficient housing, and disposable, single-use technologies.

ODU is one of many medical connectivity components suppliers that offers customizable, overmolded, medical-grade silicone cable assemblies. The ODU-MAC WHITE-LINE (pictured above) is a versatile hybrid connector and cable assembly series with an ergonomic plastic housing that can accommodate up to 90 connection points and support hybrid combinations of 18 module varieties, including signal, power, high-current, high-voltage, coax, pneumatic and fluid, fiber optic, and high-speed modules. The series also offers non-magnetic solutions, which can be especially useful in medical applications, and all of its touchable parts are nickel-free.

Several medical connectivity component suppliers, including Fischer Connectors, LEMO, Smiths Interconnect, and Molex, are also now offering disposable connector solutions for medical applications.
The Molex Plastic Circular (MPC) Interconnect System (pictured to the right) provides reliable, affordable alternatives to typical medical circular connectors in applications including disposable sensors and catheters, dental, electrophysiology, imaging, and surgical equipment, patient monitoring devices, and telehealth systems. Part of the MediSpec Product Family, the MPC Interconnect System offers custom-off-the-shelf plugs, receptacles, pigtails, and cable assemblies. The system supports selective plating for further cost reductions and ensures a reliable electrical interface for a minimum of 10,000 mating cycles.
Summation
Safety and reliability are of the utmost importance when it comes to medical connectivity components, and especially so as medical equipment continues to scale down in size and up in connectivity and capabilities. Carefully considering the six core characteristics addressed here when specifying medical connectivity components can help ensure that selected connectors and cable assemblies satisfy the rigorous form, fit, safety, and performance requirements that next-generation medical applications demand.





