Disposable Medical Devices Increase Safety for Patients
Disposable and limited reuse medical devices pack technology into a cost-effective format that can be a viable alternative to reusable devices, particularly when it comes to preventing contamination. Cost, functionality, and environmental impact factor into the choice of device as well.
Patient safety is a top priority for medical device makers and their customers. Limiting the risk of contamination and infection is a major factor in maintaining that safety, and, by extension, in designing and selecting the devices used to provide patient care. The world of disposable medical devices is constantly evolving, due in large part to the need for sterilization, according to Justin Fry, senior product line manager at Smiths Interconnect.
Sterilizing reusable equipment and devices is not an easy process. The most common method is ethylene oxide gas (EtO) sterilization. EtO came into widespread use due to its ability to sterilize heat- and moisture-sensitive medical devices. The basic EtO sterilization cycle has five stages and takes approximately 2 1/2 hours followed by aeration time (as long as 8 to 12 hours) for the desorption of residual EtO in exposed absorbent materials. It is still one of the most convenient methods because it can be done in very large gas chambers. “Some companies have chambers, the size of railcars, and they can just roll in pallets of devices and sterilize them all at once. So, it’s a very cost effective and thorough means of sterilization,” said Fry.
Despite its wide acceptance, EtO is closely regulated by the EPA. “The EPA has stepped in to create more regulations on how much exhaust can be put off by these sterilization systems and is constantly adjusting its guidance and requirements,” said Fry. “One of the big challenges is finding alternate methods that can suitably sterilize equipment. Every sterilization method has risks and benefits.” Steam is one alternative, but it ruins electronic components and certain types of plastics. Similarly, gamma radiation exposure can affect how plastics perform long term.
These issues are driving demand for the development of single-use and limited-reuse devices, where that is feasible, said Fry. Single-use devices lower the risk of infection particularly in the case of superbugs, strains of bacteria, viruses, parasites, and fungi that are resistant to antibiotics and other medications commonly used to treat the infections they cause. That benefit must be weighed against the ease of making something disposable while maintaining high functionality at a cost that can justify discarding it after one use. Reusable devices are expensive, but their cost-per-therapy is low because they can be sterilized and reused dozens of times.

Eclipta series connectors from Smiths Interconnect embed a high-performance edge card technology for disposable medical applications. For electrophysiology catheter applications, Eclipta series connectors bridge the gap between the catheter and the extension cable. A standout feature of these connectors is the fact that the PCB acts as the contact in the connector. Since the board is often part of the disposable device, it eliminates the cost of the contact system on that side.
The endoscope is a prime example of a reusable device that has gained success as a disposable. Because it goes into the body, the risk of contamination and infection is high. A single-use version greatly reduces that risk, while also eliminating the environmental concerns of EtO sterilization. Endoscopes and other similar devices contain advanced imaging technology, including an image sensor, optical lens, and light source. The goal is to maintain enough of the technology for the device to be useful to the physician and provide the best possible patient outcome but be affordable enough to discard after a single use.
As disposable medical devices gain acceptance, the issue of how to dispose of them and what impacts that has on the environment come into play. This has spurred the rise of third-party reprocessing companies that repurpose the materials or create new devices from parts of disposable devices.
“How the public thinks, what the public needs, and what regulations governing agencies implement drive OEMs to be very creative and innovative in their product design. FDA regulations, EPA restrictions, Reach and RoHS compliance, the use of hazardous materials or conflict minerals all impact the creation of a device that delivers a high level of care — one that allows patients the shortest hospital stay and the least amount of discomfort possible,” said Fry.
Limited-reuse devices
A sort of mid-ground between reusable and disposable is limited reuse. Limited reuse devices can be used for a specified number of times. That number is determined by how often the device can be sterilized and still work. The OEMs design and test the device to withstand sterilization and work properly for many more uses than they rate them for, knowing that, if the device works and can be sterilized, there is a chance it will continue to be used. This is a particular issue in low-resource facilities around the world. “Many manufacturers are now putting an EPROM inside the connector or cable in the device to track the number of times it has been used and indicate when it should be discarded,” Fry said.
In some cases, a system can have a combination of single-use and limited-reuse components. “We do a lot in the electrophysiology market. There’s the catheter side that enters the patient’s body that is single use, one catheter per patient. Coming out the backside of that catheter is an electrical interconnect, basically a cable that transmits signal or energy between the catheter and the mapping system or generator, to create a cardiac map or deliver ablation therapy,” said Fry. “The cable is typically in the sterile field, but because it can be expensive to make it is rated to be sterilized and reprocessed. So, while the catheter and the catheter handle are single use, the cable is considered limited reuse and can be sterilized for the number of times specified by the manufacturer.”
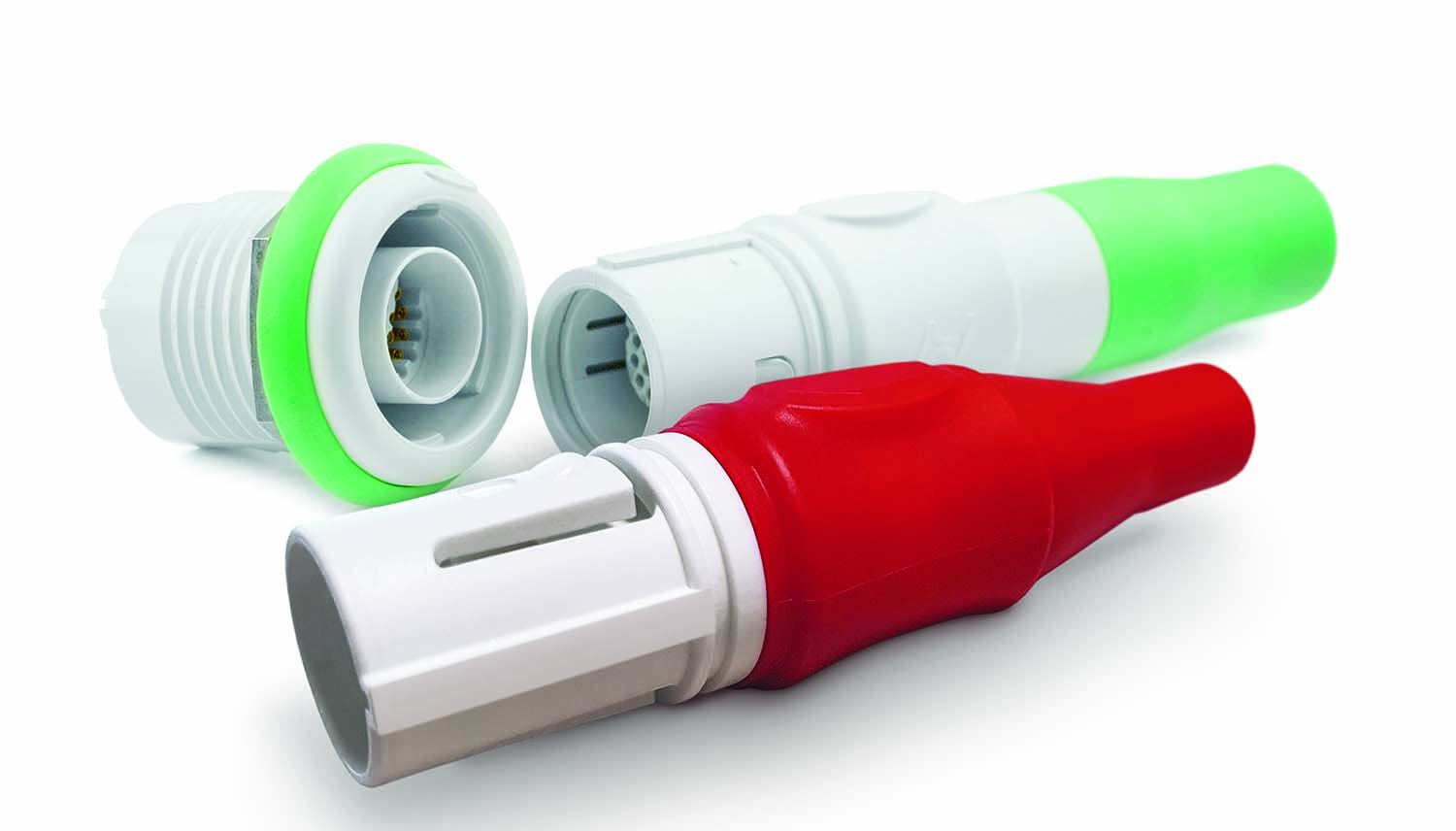
Hypergrip series disposable connector plugs from Smiths Interconnect deliver a reliable interconnect solution for single or few uses, ideal for applications that don’t require high cycle life but still require a premium disposable connector mated with a high cycle receptacle. The disposable plugs maintain the premium look-and-feel, reliability, compliance with medical industry standards, and ease of use of the standard Hypergrip series connectors and are designed to support overmolding and high-volume production.
Connectors and disposable medical device design
Medical device manufacturers have varying degrees of knowledge about the interconnects they need and typically rely on the connector company subject matter experts to help them develop their products. Fry explained that what connector suppliers are asked to do is a mixed bag, depending on how much experience the OEMs have and what they are developing. If they’re designing the next generation of an existing system, they’ll try to keep it in the same form factor with only minor adjustments because it is easier for their process of qualification.
The reality is that the connector itself is sometimes an afterthought for the OEMs. “When our OEMs are creating a new device, we ask them to engage us as early as they would any supplier they’re relying on for any other part of that device. The earlier we get involved, the more robust solution we can provide, because it gives us time to provide samples, receive feedback, and iterate with the proposed design.”
Two key requirements that are becoming more evident in disposable medical device design are higher voltage ratings and higher densities. “Some new therapies are starting to enter the market, particularly in electrophysiology. For example, there are new ablation technologies that are showing promising results in both safety and efficacy of tissue ablation, but they require higher voltages,” said Fry. “Now we have to think through how to increase the content inside of a connector, allowing it to now include high-voltage capability, which requires us to think of new ways to configure and insulate the contacts to deliver the required performance and safety while maintaining both the size and mating forces that are ergonomic for the user.”
The demand for higher density connectors — fitting more functionality in the same footprint, has been steadily increasing. “That drives us to look at alternate materials and contact technologies. For the electrophysiology and imaging markets we’re looking at fitting well over one hundred contact positions within a package that can fit inside a catheter handle, giving us just over an inch in diameter to work with.” In the imaging market, where image quality is paramount, higher density allows for more channels to help process and deliver more data for a faster, clearer image.
Visit the Preferred Supplier page for Smiths Interconnect to learn more about the company and its products.
Like this article? Check out our other Technology Trends articles, our Medical Market Page, and our 2023 and 2024 Article Archives.
Subscribe to our weekly e-newsletters, follow us on LinkedIn, Twitter, and Facebook, and check out our eBook archives for more applicable, expert-informed connectivity content.
- Sealing Success: Overmolding for More Secure Connections - April 23, 2024
- Medical Cable Assemblies Product Roundup - April 23, 2024
- Mezzanine Connectors Product Roundup - April 16, 2024